10.1021/jo0606655
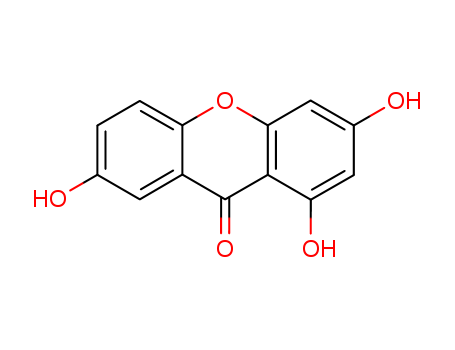
The research aims to develop a simple and efficient method for synthesizing 1,3,7-trihydroxyxanthone and its subsequent regioselective coupling reactions with prenal to exclusively produce the natural products osajaxanthone and nigrolineaxanthone F. The study describes a five-step synthesis of 1,3,7-trihydroxyxanthone starting from 1,3,5-trimethoxybenzene, involving NBS-induced bromination, lithiation, benzoylation, selective deprotection, intramolecular cyclization, and demethylation, achieving an overall yield of 62%. The regioselective coupling reactions with prenal were conducted under different conditions: at room temperature with calcium hydroxide yielding osajaxanthone (75% yield) and under thermal conditions at 140-150 °C yielding nigrolineaxanthone F (98% yield). The study concludes that this approach offers a straightforward and efficient route to these xanthones, with high yields and selectivity, providing a significant improvement over previous methods. The structures of the compounds were confirmed using analytical and spectral data, including X-ray crystallography.