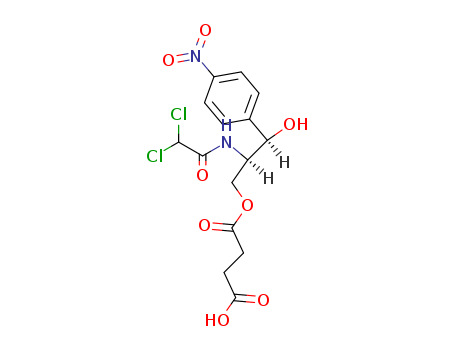
- Chemical Name:Chloramphenicol succinate
- CAS No.:3544-94-3
- Deprecated CAS:53906-65-3
- Molecular Formula:C15H16 Cl2 N2 O8
- Molecular Weight:423.207
- Hs Code.:
- European Community (EC) Number:222-590-0
- NSC Number:756676
- UNII:ZCX619U9A1
- DSSTox Substance ID:DTXSID8048155
- Wikidata:Q27096784
- Metabolomics Workbench ID:69973
- ChEMBL ID:CHEMBL1201281
- Mol file:3544-94-3.mol
Synonyms:chloramphenicol hemisuccinate;chloramphenicol monosuccinate;chloramphenicol sodium succinate;chloramphenicol succinate;chloramphenicol succinate sodium;levomycetin succinate