10.1002/anie.201600379
The research aims to develop a highly enantioselective method for synthesizing chiral 1,4-benzodioxanes, 1,4-benzooxazines, and chromans, which are important structural units in many bioactive natural products and drugs. The study focuses on using palladium-catalyzed alkene aryloxyarylation reactions, with key chemicals including 2-((2-methylallyl)oxy)phenol (1a), various aryl halides such as bromobenzene (2a), and chiral monophosphorus ligands like L4 and L5. The researchers optimized the reaction conditions, finding that a strong base like NaOtBu and a solvent like hexafluorobenzene (C6F6) enhanced both yield and enantioselectivity. The method demonstrated high yields (up to 90%) and excellent enantioselectivity (up to 95% ee) for a range of substrates, including those with different aryl and heteroaryl groups. The study concludes that the chiral monophosphorus ligands L4 and L5 are crucial for the high reactivity and enantioselectivity of the transformations. The findings not only provide a practical route for synthesizing these chiral compounds but also offer valuable insights into the design of better catalytic systems for similar transformations.
10.1021/ja00072a046
The research investigates the intermolecular fluorine atom abstraction from fluorocarbons, including saturated perfluorocarbons, by the compound (MeCsH&U(t-Bu). The study reports that this compound can efficiently abstract fluorine atoms under mild conditions in hydrocarbon solvents. Key chemicals involved in the research include hexafluorobenzene, which reacts with (MeCsH&U(t-Bu) to produce uranium (IV) fluoride and various organic products such as CsFsH, CsFs(t-Bu), isobutane, and isobutene. Other fluorocarbons like benzotrifluoride and perfluoromethylcyclohexane were also used to explore the scope of the C-F bond activation process. The reactions were monitored using techniques like NMR spectroscopy, GC, and GC-MS to identify and quantify the products. The study proposes a radical cage mechanism for these reactions, suggesting that the formation of a U-F bond and subsequent C-C or C-H bond formations drive the process.
10.1039/DT9930003097
The research investigates the synthesis and properties of sandwich complexes of tungsten and molybdenum containing hexafluorobenzene (C6F6) or 1,3,5-trifluorobenzene (C6H3F3-1,3,5) ligands. The purpose is to explore the reactions of metal atoms with potentially oxidising ligands and extend the known role of hexafluorobenzene as a ligand. Key chemicals used include molybdenum or tungsten atoms, hexafluorobenzene, 1,3,5-trifluorobenzene, benzene, and 1,3,5-trimethylbenzene. The researchers found that reacting molybdenum or tungsten atoms with these ligands at liquid-nitrogen temperature produced new compounds such as [M(C6F6)2], [M(C6H3F3-1,3,5)2], and mixed-ligand complexes like [M(C6F6)(C6H6)] and [M(C6F6)(C6H3Me3-1,3,5)]. The study revealed that these complexes are stable and resistant to air oxidation, with bond lengths and angles indicating typical metal-arene bonding. The 19F and 1H NMR spectra showed significant inter-ring coupling, making some spectra easier to interpret. The research concludes that hexafluorobenzene can form stable complexes with transition metals, and this finding could stimulate further conventional organometallic synthesis in this area.

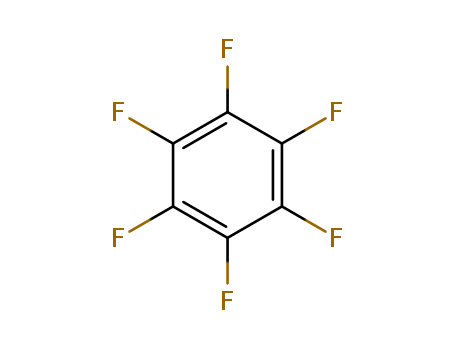

 F,
F,  Xi
Xi


