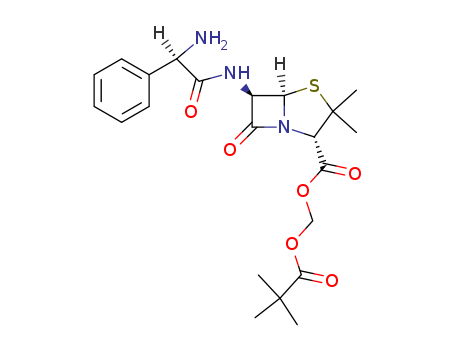
- Chemical Name:Pivampicillin
- CAS No.:33817-20-8
- Molecular Formula:C22H29 N3 O6 S
- Molecular Weight:463.555
- Hs Code.:
- European Community (EC) Number:251-688-6
- UNII:0HLM346LL7
- DSSTox Substance ID:DTXSID1045459
- Nikkaji Number:J17.773C
- Wikipedia:Pivampicillin
- Wikidata:Q3122143
- NCI Thesaurus Code:C84068
- Metabolomics Workbench ID:43591
- ChEMBL ID:CHEMBL3182343
- Mol file:33817-20-8.mol
Synonyms:Ampicillin Pivaloyl Ester;Berocillin;Ester, Ampicillin Pivaloyl;Hydrochloride, Pivampicillin;Monohydrochloride, Pivampicillin;Pivamiser;Pivampicillin;Pivampicillin Hydrochloride;Pivampicillin Monohydrochloride;Pondocillin