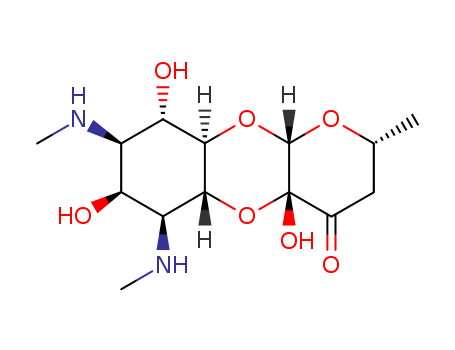
- Chemical Name:Spectinomycin
- CAS No.:1695-77-8
- Molecular Formula:C14H24N2O7
- Molecular Weight:332.354
- Hs Code.:3003209000
- European Community (EC) Number:244-554-3,216-911-3,606-950-8
- UNII:93AKI1U6QF
- DSSTox Substance ID:DTXSID9023592
- Nikkaji Number:J7.562K
- Wikipedia:Spectinomycin
- Wikidata:Q416154
- NCI Thesaurus Code:C61951
- Metabolomics Workbench ID:43186
- ChEMBL ID:CHEMBL1167
- Mol file:1695-77-8.mol
Synonyms:Actinospectacin;Adspec;Ferkel Spectam;Kempi;Prospec;Salmosan T;Salmosan-T;Spectam;Spectam, Ferkel;Spectinomycin;Spectinomycin Dihydrochloride, Anhydrous;Spectinomycin Dihydrochloride, Pentahydrate;Spectinomycin Hydrochloride;Stanilo;Trobicin