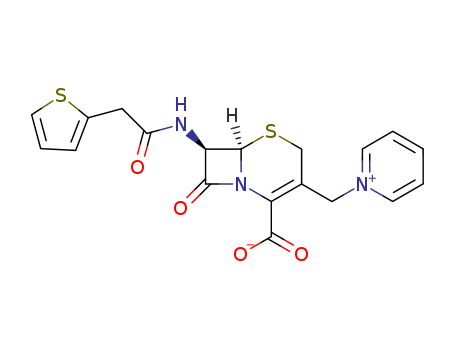
- Chemical Name:Cephaloridine
- CAS No.:50-59-9
- Molecular Formula:C19H17 N3 O4 S2
- Molecular Weight:415.494
- Hs Code.:
- European Community (EC) Number:200-052-6
- UNII:LVZ1VC61HB
- DSSTox Substance ID:DTXSID9022782
- Wikipedia:Cephaloridine
- Wikidata:Q5063323
- NCI Thesaurus Code:C76594
- Pharos Ligand ID:6XX8T3F4UTX3
- Metabolomics Workbench ID:49984
- ChEMBL ID:CHEMBL316157
- Mol file:50-59-9.mol
Synonyms:Cefaloridine;Cephalomycine;Cephaloridin;Cephaloridine;Ceporin