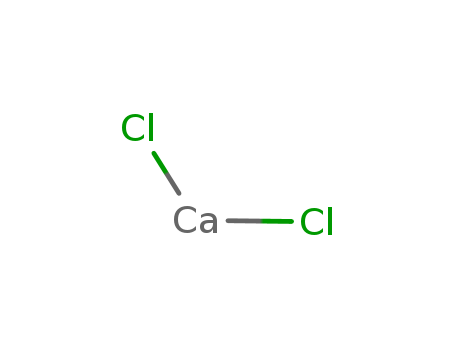
- Chemical Name:Calcium chloride ca-45
- CAS No.:14336-71-1
- Molecular Formula:CaCl2
- Molecular Weight:110.984
- Hs Code.:
- UNII:TIW7SL1DSX
- DSSTox Substance ID:DTXSID60931841
- Wikidata:Q27289986
- NCI Thesaurus Code:C87197
- ChEMBL ID:CHEMBL2106539
- Mol file:14336-71-1.mol
Synonyms:calcium chloride ca-45;Calcium chloride, ca-45;(~45~Ca)Calcium dichloride;CHEMBL2106539;DTXSID60931841;CALCIUM CHLORIDE ((SUP 45)CACL(SUB 2));Q27289986