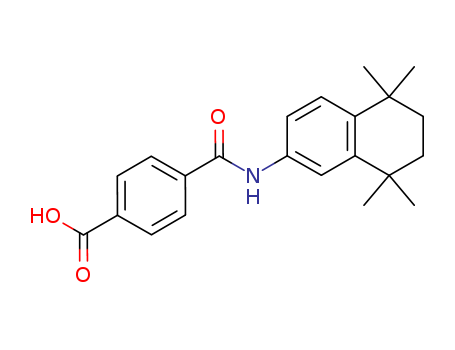
- Chemical Name:Tamibarotene
- CAS No.:94497-51-5
- Molecular Formula:C22H25NO3
- Molecular Weight:351.445
- Hs Code.:2924299090
- NSC Number:608000
- UNII:08V52GZ3H9
- DSSTox Substance ID:DTXSID5046853
- Nikkaji Number:J227.635F
- Wikipedia:Tamibarotene
- Wikidata:Q7681221
- NCI Thesaurus Code:C71025
- Pharos Ligand ID:7CLT73A6ZVBQ
- Metabolomics Workbench ID:43645
- ChEMBL ID:CHEMBL25202
- Mol file:94497-51-5.mol
Synonyms:4-((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl)benzoic acid;Am 80;AM-80;Am80;tamibarotene