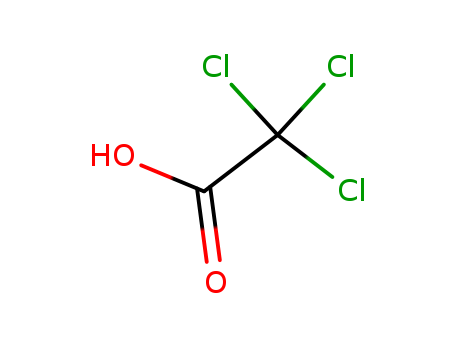
- Chemical Name:Trichloroacetic Acid
- CAS No.:76-03-9
- Molecular Formula:C2HCl3O2
- Molecular Weight:163.388
- Hs Code.:2915.40
- European Community (EC) Number:200-927-2
- ICSC Number:0586
- NSC Number:215204,77363
- UN Number:1839,2564
- UNII:5V2JDO056X
- DSSTox Substance ID:DTXSID1021378
- Nikkaji Number:J2.400G
- Wikipedia:Trichloroacetic_acid
- Wikidata:Q410116
- Metabolomics Workbench ID:49716
- ChEMBL ID:CHEMBL14053
- Mol file:76-03-9.mol
Synonyms:Acid, Trichloroacetic;Acide trichloracetique;Rubidium Trichloroacetate;Sodium Trichloroacetate;trichloracetique, Acide;Trichloroacetate, Rubidium;Trichloroacetate, Sodium;Trichloroacetic Acid


 Xn,
Xn,  N,
N,  C,
C,  F
F
 C:Corrosive;
C:Corrosive;

