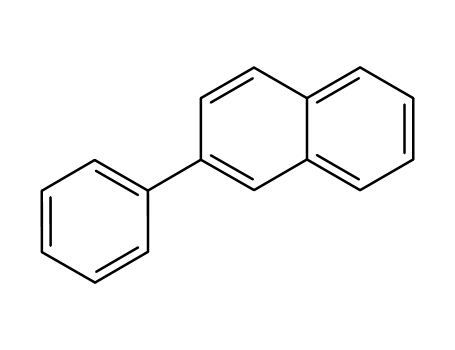
- Chemical Name:2-Phenylnaphthalene
- CAS No.:612-94-2
- Molecular Formula:C16H12
- Molecular Weight:204.271
- Hs Code.:29334990
- European Community (EC) Number:210-324-6
- NSC Number:407592
- UNII:949VN5DH7A
- DSSTox Substance ID:DTXSID8060614
- Nikkaji Number:J117.144E
- Wikidata:Q27271663
- Mol file:612-94-2.mol
Synonyms:2-PHENYLNAPHTHALENE;612-94-2;Naphthalene, 2-phenyl-;.beta.-Phenylnaphthalene;Naphthalene, phenyl-;beta-Phenylnaphthalene;NSC 407592;UNII-949VN5DH7A;949VN5DH7A;EINECS 210-324-6;NSC-407592;35465-71-5;2-Phenylnaphthalene 10 microg/mL in Cyclohexane;2-Phenylnaphthalin;6-phenylnaphthalene;2-phenyl-naphthalene;c1(ccc2ccccc2c1)c3ccccc3;DTXSID8060614;MFCD00039601;NSC407592;AKOS016015924;SS-4770;CS-0269833;FT-0632555;P0679;T72706;A868752;Q27271663;Z1723549920


 Xi,
Xi, Xn
Xn


