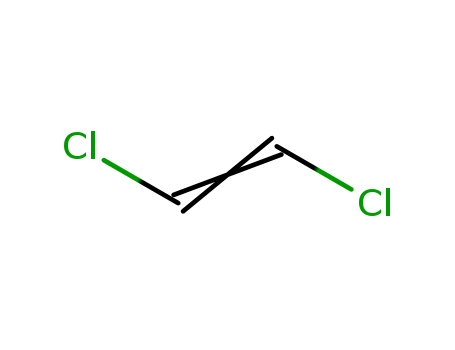
- Chemical Name:1,2-Dichloroethylene
- CAS No.:540-59-0
- Molecular Formula:C2H2Cl2
- Molecular Weight:96.9439
- Hs Code.:2903299090
- European Community (EC) Number:208-750-2
- ICSC Number:0436
- UN Number:1150
- DSSTox Substance ID:DTXSID8024991
- Wikipedia:1,2-Dichloroethene
- Wikidata:Q161475
- ChEMBL ID:CHEMBL1385560
- Mol file:540-59-0.mol
Synonyms:1,2-dichloroethene;1,2-dichloroethylene;1,2-dichloroethylene, (E)-isomer;1,2-dichloroethylene, (Z)-isomer


 F,
F,  Xn
Xn


