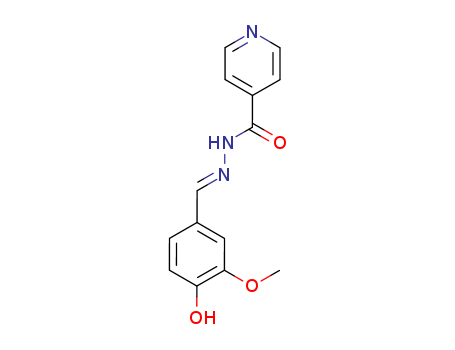
- Chemical Name:Phthivazid
- CAS No.:149-17-7
- Molecular Formula:C14H13 N3 O3
- Molecular Weight:271.276
- Hs Code.:2933399090
- UNII:40Q4C3O4V0
- DSSTox Substance ID:DTXSID3020758
- Wikidata:Q4493190
- NCI Thesaurus Code:C72604
- Pharos Ligand ID:LG2N9C43FBHG
- Mol file:149-17-7.mol
Synonyms:Acid Vanillylidenehydrazide, Isonicotinic;Ftivazide;Hydrazide, Isonicotinic Acid;Isonex;Isoniazid;Isonicotinic Acid Hydrazide;Isonicotinic Acid Vanillylidenehydrazide;phthivazid;Phthivazide;Tubazide;Vanillylidenehydrazide, Isonicotinic Acid