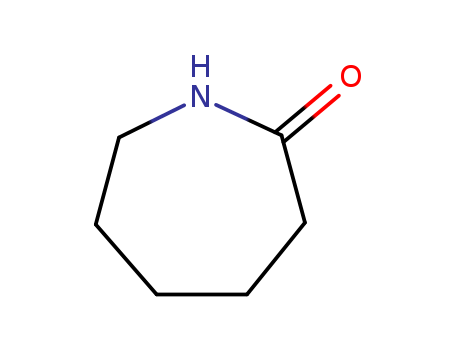
- Chemical Name:Caprolactam
- CAS No.:105-60-2
- Deprecated CAS:117955-36-9,168214-28-6,2953-03-9,32838-21-4,32838-23-6,34876-18-1,168214-28-6,2953-03-9,32838-21-4,34876-18-1
- Molecular Formula:C6H11NO
- Molecular Weight:113.159
- Hs Code.:2933710000
- European Community (EC) Number:919-537-5
- ICSC Number:0118
- NSC Number:117393,25536,4977
- UN Number:3082
- UNII:6879X594Z8
- DSSTox Substance ID:DTXSID4020240
- Nikkaji Number:J3.605F
- Wikipedia:Caprolactam
- Wikidata:Q409397
- Metabolomics Workbench ID:51773
- ChEMBL ID:CHEMBL276218
- Mol file:105-60-2.mol
Synonyms:Aminocaproic Lactam;Caprolactam;Hexahydro 2H Azepin 2 One;Hexahydro-2H-Azepin-2-One;Lactam, Aminocaproic


 Xn
Xn
 Xn:Harmful;
Xn:Harmful;

