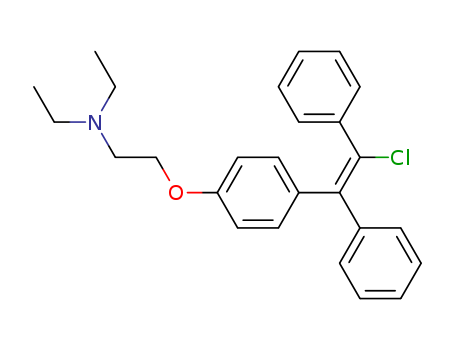
- Chemical Name:Clomifene
- CAS No.:15690-55-8
- Molecular Formula:C26H28ClNO
- Molecular Weight:405.967
- Hs Code.:2922299090
- European Community (EC) Number:213-008-6
- UNII:3JU1DU3652
- DSSTox Substance ID:DTXSID601317947
- Nikkaji Number:J7.163C,J9.660A,J9.012C
- Wikipedia:Clomifene,Zuclomifene
- Wikidata:Q27257344
- NCI Thesaurus Code:C66870
- RXCUI:2596
- Pharos Ligand ID:JCJU3BZRM8FS
- Metabolomics Workbench ID:124125
- ChEMBL ID:CHEMBL167779
- Mol file:15690-55-8.mol
Synonyms:Chloramiphene;Citrate, Clomiphene;Clomid;Clomide;Clomifen;Clomifene;Clomiphene;Clomiphene Citrate;Clomiphene Hydrochloride;Clostilbegit;Dyneric;Gravosan;Hydrochloride, Clomiphene;Klostilbegit;Serophene