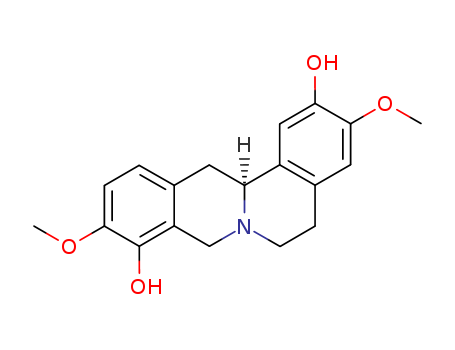
- Chemical Name:(S)-Scoulerine
- CAS No.:6451-73-6
- Molecular Formula:C19H21NO4
- Molecular Weight:327.38
- Hs Code.:
- Nikkaji Number:J92.838K
- Wikipedia:Scoulerine
- Wikidata:Q7438285
- Pharos Ligand ID:NCU8RTWKKGWA
- Metabolomics Workbench ID:50787
- ChEMBL ID:CHEMBL1235966
- Mol file:6451-73-6.mol
Synonyms:aequaline;aequaline, (S)-isomer;discretamine;discretamine, (+-)-isomer;discretamine, (R)-isomer;discretamine, (S)-isomer;discretamine, hydrochloride, (S)-isomer;scoulerine;scoulerine, (S)-isomer