10.1021/ja01606a062
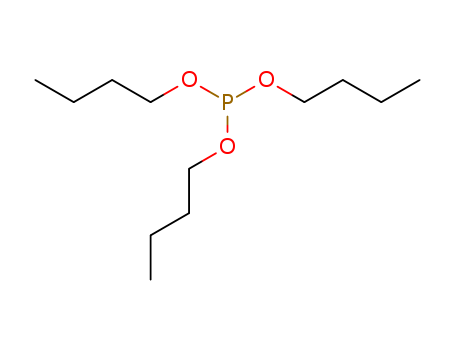
The study investigates the reaction of sulfenyl chlorides with trialkyl phosphites, resulting in the formation of esters of monothiophosphoric acid. Various alkyl and aromatic sulfenyl chlorides, such as methanesulfenyl chloride, benzenesulfenyl chloride, and p-chloroethanesulfenyl chloride, were reacted with triethyl phosphite, tri-n-propyl phosphite, and tri-n-butyl phosphite. The reactions were rapid, even at Dry Ice temperatures, indicating a nucleophilic displacement of chloride accompanied by the elimination of alkyl chloride. The study also compared the reactivity of these sulfenyl chlorides with that of sulfur monochloride and noted that the sulfenyl chlorides reacted at least as readily as acyl halides, which are known to react exothermally with tertiary phosphites. The compounds synthesized were used for biological testing in cancer chemotherapy studies, with particular interest in the 6-chloro thioester as a potential mustard analog.