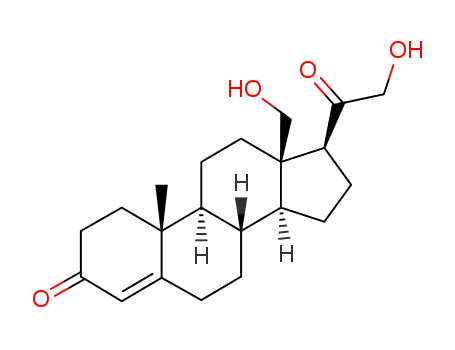
- Chemical Name:18,21-Dihydroxypregn-4-ene-3,20-dione
- CAS No.:379-68-0
- Molecular Formula:C21H30O4
- Molecular Weight:346.467
- Hs Code.:
- European Community (EC) Number:206-834-3
- DSSTox Substance ID:DTXSID90958920
- Nikkaji Number:J117.990J
- Wikipedia:18-Hydroxy-11-deoxycorticosterone
- Wikidata:Q82939478
- Mol file:379-68-0.mol
Synonyms:18,21-dihydroxy-4-pregnene-3,20-dione;18-hydroxydeoxycorticosterone