10.1016/S0040-4039(00)84436-6
The research focused on the structure and synthesis of WF 3681, a novel aldose reductase inhibitor isolated from a Chaetomella species. The purpose of the study was to elucidate the structure of WF 3681 and confirm it through total synthesis. The researchers concluded that WF 3681, a fungal metabolite, possesses potent aldose reductase-inhibitory activity and has the chemical structure C13H1205. Key chemicals used in the synthesis process included (E)-5-phenyl-4-pentenol, benzyl bromide, MCPBA (m-chloroperoxybenzoic acid), methyl malonate, NaOH (sodium hydroxide), CH2O/Me2NH (formaldehyde/dimethylamine), Os04-NaI04 (osmium tetroxide-sodium periodate), Pd-black (palladium on carbon), EtOCOCl (ethyl chloroformate), Cr03 (chromium trioxide), and K2CO3 (potassium carbonate). The synthesis involved a series of reactions, including protection of hydroxy groups, oxidation, regiospecific opening of epoxide rings, alkaline hydrolysis, formation of Mannich bases, and oxidation to form the final product, which was confirmed to be identical to the natural product WF 3681.
10.1248/cpb.34.423
The research aimed to develop a facile synthesis method for 6,3'-methanouridine and 6,3'-methanocytidine, which are nucleosides with a methylene group bridging the C-6 of the sugar and the 3'-position of the pyrimidine base. These compounds are of interest for their potential biological and pharmacological properties. The synthesis involved the condensation of 2,4-dimethoxy-6-lithiomethylpyrimidine with 5-O-(tert-butyldimethyl)silyl-1,2-O-isopropylidene-3-ketoxylose, followed by intramolecular glycosylation and deprotection steps. Key chemicals used in the process included 1,2-O-isopropylidene-D-xylose, t-butyldimethylchlorosilane, chromium trioxide, tetrahydrofuran (THF), and various protecting groups such as t-butyldimethylsilyl and benzoyl groups. The researchers concluded that their method was effective for the large-scale preparation of 6,3'-methanouridine and related compounds of biochemical interest.
10.3987/COM-13-S(S)15
The study presents a new, environmentally friendly synthesis method for tofisopam, a 2,3-benzodiazepine type anxiolytic drug, which avoids the use of toxic chromium(VI) oxide reagents traditionally used in its production. The research focuses on replacing the hazardous chromium-based oxidation step with a lithiation-based approach to produce the key intermediate, a keto-ketal compound. The chemicals used in the study include (3,4-dimethoxyphenyl)acetone, bromine, lithium compounds (hexyllithium), 3,4-dimethoxybenzoyl chloride, and hydrazine, among others. These chemicals serve various purposes in the synthesis process, such as starting materials, reagents for protection and deprotection of functional groups, and agents for the lithiation and cyclization steps, ultimately leading to the formation of tofisopam without the environmental and health risks associated with chromium salts.
10.1016/S0040-4039(00)84713-9
The study investigates the synthesis and antibacterial activity of various derivatives of penicillin. The researchers aimed to create 6a-carboxy and 6a-carbamoyl penicillins, which are structural analogues of previously studied 6a-(hydroxymethyl) and 6a-formyl penicillins known for their stability to β-lactamases and antibacterial activity. Key chemicals involved include 6a-(hydroxymethyl)penicillanate and 6a-formyl penicillin, which served as starting materials. The researchers used reagents such as t-butyl alcohol, chromium trioxide, pyridine, acetic anhydride, and allyl alcohol to oxidize and esterify these compounds, ultimately synthesizing various penicillin carboxylates. They also attempted to prepare a 6a-carbamoyl penicillin using mixed anhydride formation and ammonia. Despite successful synthesis of several derivatives, including the allyl ester and pivaloyloxymethyl ester, the final penicillin products exhibited poor antibacterial activity.


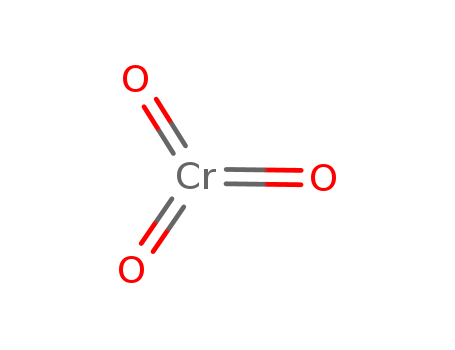
 O,
O, T+,
T+, N
N
 O:Oxidizing agent;
O:Oxidizing agent;

