Suppliers and Price of Chromium
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- Sigma-Aldrich
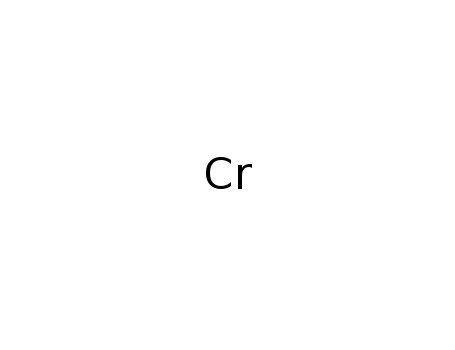
- Chromium
foil, light tested, 25x25mm, thickness 0.015mm, permanent polyester support, 99.99+%
- 2ea
- $ 957.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 50x50mm, thickness 0.008mm, permanent polyester support, 99.99+%
- 1ea
- $ 961.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 25x25mm, thickness 0.04mm, permanent polyester support, 99.99+%
- 2ea
- $ 965.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 25x25mm, thickness 0.002mm, permanent polyester support, 99.99+%
- 2ea
- $ 967.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 50x50mm, thickness 0.007mm, permanent polyester support, 99.99+%
- 1ea
- $ 967.00
- Sigma-Aldrich
- Chromiumfoil, not light tested, 50x50mm, thickness 0.0125mm, permanent polyester support, 99.99+%
- 1ea
- $ 968.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 25x25mm, thickness 0.05mm, permanent polyester support, 99.99+%
- 2ea
- $ 973.00
- Sigma-Aldrich
- Chromium
foil, thickness 1.0 mm, size 12.5 × 12.5 mm, purity 99.99%
- 2ea
- $ 978.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 25x25mm, thickness 0.0015mm, permanent polyester support, 99.99+%
- 2ea
- $ 989.00
- Sigma-Aldrich
- Chromium
foil, not light tested, 50x50mm, thickness 0.006mm, permanent polyester support, 99.99+%
- 1ea
- $ 1010.00
-
Total 145 raw suppliers