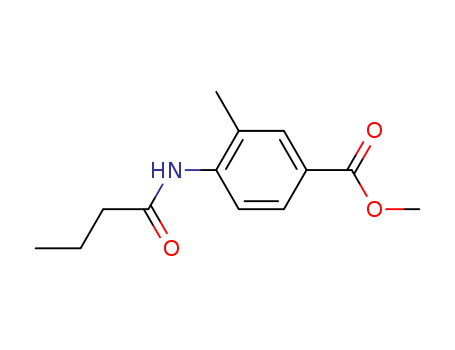
- Chemical Name:Methyl 4-butyramido-3-methylbenzoate
- CAS No.:301533-59-5
- Molecular Formula:C13H17NO3
- Molecular Weight:235.283
- Hs Code.:2924299090
- DSSTox Substance ID:DTXSID80431858
- Wikidata:Q72488962
- Mol file:301533-59-5.mol
Synonyms:Methyl 4-butyramido-3-methylbenzoate;301533-59-5;methyl 4-(butanoylamino)-3-methylbenzoate;SCHEMBL3789228;DTXSID80431858;RZMQQYDXKPDLJH-UHFFFAOYSA-N;Benzoic acid, 3-methyl-4-[(1-oxobutyl)amino]-, methyl ester;Methyl4-butyramido-3-methylbenzoate;MFCD21368226;AKOS009141931;methyl 4-butyrylamino-3-methylbenzoate;AC-6860;AM90300;DS-17765;methyl N-butyryl-4-amino-3-methylbenzoate;methyl N-butyryl-4-amino-3-methyl-benzoate;CS-0152461;FT-0658737;D78269;3-Methyl-4-butyrylaminobenzoic acid methyl ester;4-Butyrylamino-3-methylbenzoic acid methyl ester;Methyl 4-Butyrylamino-3-methylbenzoate