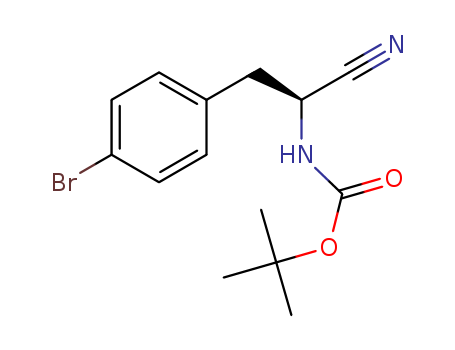
- Chemical Name:BOC-L-4-BR-PHE-NITRILE
- CAS No.:869570-00-3
- Molecular Formula:C14H17 Br N2 O2
- Molecular Weight:325.205
- Hs Code.:
- Mol file:869570-00-3.mol
Synonyms:Carbamicacid, [(1S)-2-(4-bromophenyl)-1-cyanoethyl]-, 1,1-dimethylethyl ester (9CI);(2S)-2-(tert-Butoxycarbonylamino)-3-(4-bromophenyl)propanenitrile