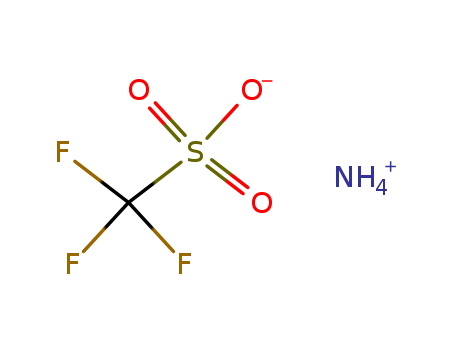
- Chemical Name:Ammonium trifluoromethanesulfonate
- CAS No.:38542-94-8
- Molecular Formula:CH F3 O3 S . H3 N
- Molecular Weight:167.109
- Hs Code.:29310099
- European Community (EC) Number:629-264-0
- DSSTox Substance ID:DTXSID50578805
- Mol file:38542-94-8.mol
Synonyms:Ammonium trifluoromethanesulfonate;38542-94-8;Ammonium triflate;azanium;trifluoromethanesulfonate;SCHEMBL137559;Ammoniumtrifluoromethanesulfonate;DTXSID50578805;MFCD00075332;D94729