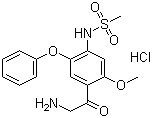
- Chemical Name:N-(4-(2-Aminoacetyl)-5-methoxy-2-phenoxyphenyl)methanesulfonamide hydrochloride
- CAS No.:149436-41-9
- Molecular Formula:C16H18N2O5S.HCl
- Molecular Weight:386.85
- Hs Code.:2935009090
- Mol file:149436-41-9.mol
Synonyms:methanesulfonamide, N-[4-(2-aminoacetyl)-5-methoxy-2-phenoxyphenyl]-, hydrochloride (1:1);N-(4-Glycyl-5-methoxy-2-phenoxyphenyl)methanesulfonamide hydrochloride (1:1);2-Amino-1-(2-methoxy-4-methanesulfonylamino-5-phenoxyphenyl)ethanone hydrochloride;