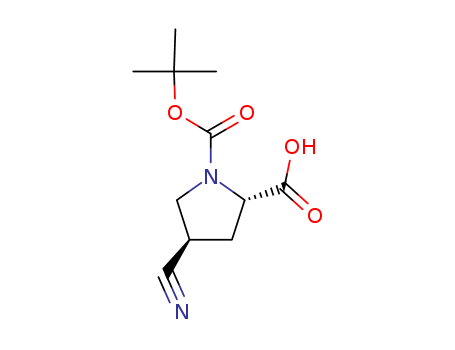
- Chemical Name:(2S,4R)-1-(tert-butoxycarbonyl)-4-cyanopyrrolidine-2-carboxylic acid
- CAS No.:273221-94-6
- Molecular Formula:C11H16 N2 O4
- Molecular Weight:240.25600
- Hs Code.:2933998090
- European Community (EC) Number:688-951-3
- DSSTox Substance ID:DTXSID20590519
- Wikidata:Q72510101
- Mol file:273221-94-6.mol
Synonyms:273221-94-6;(2S,4R)-1-(tert-butoxycarbonyl)-4-cyanopyrrolidine-2-carboxylic acid;N-Boc-trans-4-cyano-L-proline;(2S,4R)-4-cyano-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid;4-Cyano-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester;trans-N-Boc-4-cyano-L-proline;(S)-Boc-trans-4-cyanoproline;SCHEMBL1269658;(2S,4R)-1-[(tert-butoxy)carbonyl]-4-cyanopyrrolidine-2-carboxylic acid;DTXSID20590519;MIVXQYMYEMUMKK-YUMQZZPRSA-N;(4R)-1-Boc-4-cyano-L-proline;MFCD01860670;AKOS015969285;AS-47738;DB-067766;CS-0254826;A26549;F15964;(4R)-1-(tert-Butoxycarbonyl)-4-cyano-L-proline;J-016735