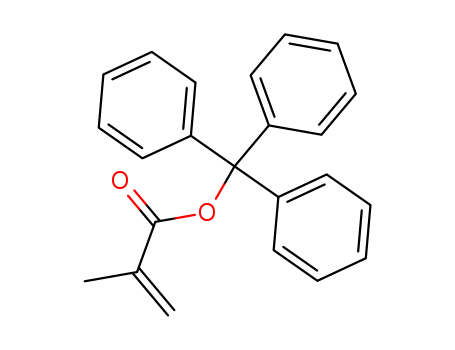
- Chemical Name:Triphenylmethyl methacrylate
- CAS No.:19302-93-3
- Molecular Formula:C23H20 O2
- Molecular Weight:328.411
- Hs Code.:2916140000
- European Community (EC) Number:662-968-6
- DSSTox Substance ID:DTXSID80505366
- Nikkaji Number:J437.676E
- Wikidata:Q82360422
- Mol file:19302-93-3.mol
Synonyms:trityl methacrylate;triphenylmethyl methacrylate;19302-93-3;trityl 2-methylprop-2-enoate;TRITYLMETHACRYLATE;SCHEMBL435821;2-Propenoic acid, 2-methyl-, triphenylmethyl ester;DTXSID80505366;PTVDYMGQGCNETM-UHFFFAOYSA-N;triphenylmethyl 2-methylprop-2-enoate;FT-0706790