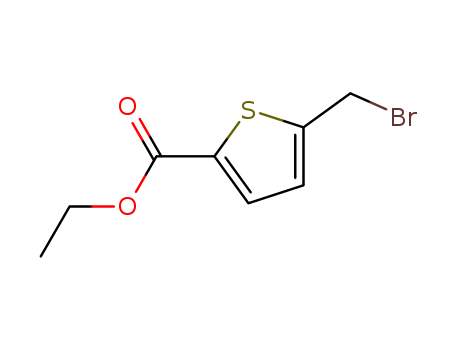
Suppliers and Price of 2-BROMOMETHYLTHIOPHENE-5-CARBOXYLIC ACID ETHYL ESTER
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- Crysdot
- Ethyl5-(bromomethyl)thiophene-2-carboxylate 95+%
- 1g
- $ 377.00
- Chemenu
- ethyl5-(bromomethyl)thiophene-2-carboxylate 95%
- 1g
- $ 356.00
- Arctom
- Ethyl5-(bromomethyl)thiophene-2-carboxylate
- 1g
- $ 353.00
- American Custom Chemicals Corporation
- 2-BROMOMETHYLTHIOPHENE-5-CARBOXYLIC ACID ETHYL ESTER 95.00%
- 5MG
- $ 501.19
- Alichem
- Ethyl5-(bromomethyl)thiophene-2-carboxylate
- 1g
- $ 429.24
-
Total 3 raw suppliers