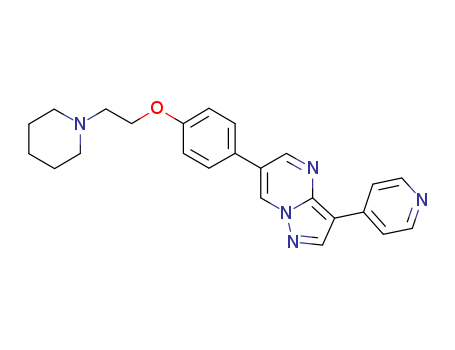
- Chemical Name:Dorsomorphin
- CAS No.:866405-64-3
- Molecular Formula:C24H25N5O
- Molecular Weight:399.495
- Hs Code.:2933990090
- European Community (EC) Number:635-949-5
- UNII:10K52CIC1Z
- ChEMBL ID:CHEMBL478629
- DSSTox Substance ID:DTXSID401006988
- Metabolomics Workbench ID:151722
- Nikkaji Number:J2.625.625I
- Pharos Ligand ID:YS5RSZQDF5R3
- Wikidata:Q27077101
- Mol file:866405-64-3.mol
Synonyms:(6-(4-(2-piperidin-1-ylethoxy)phenyl))-3-pyridin-4-ylpyrazolo(1,5-a)pyrimidine;4-(6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)pyrazolo(1,5-a)pyrimidin-3-yl)pyridine;6-(4-(2-(1-Piperidinyl)ethoxy)phenyl)-3-(4-pyridinyl)pyrazolo(1,5-a)pyrimidine;AMPK inhibitor, compound C;compound C dorsomorphin;dorsomorphin


 Xn
Xn


