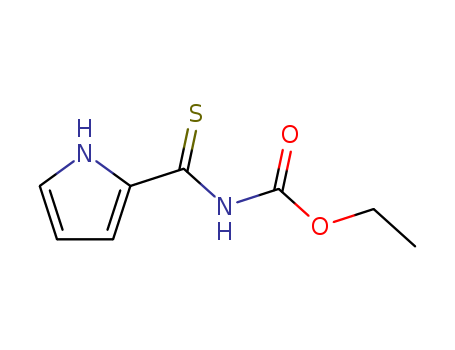
- Chemical Name:ethyl N-(1H-pyrrole-2-carbothioyl)carbamate
- CAS No.:37488-43-0
- Molecular Formula:C8H10N2O2S
- Molecular Weight:198.246
- Hs Code.:
- NSC Number:247000
- DSSTox Substance ID:DTXSID90353375
- ChEMBL ID:CHEMBL1577380
- Mol file:37488-43-0.mol
Synonyms:37488-43-0;ethyl N-(1H-pyrrole-2-carbothioyl)carbamate;ethyl 1H-pyrrol-2-ylcarbothioylcarbamate;Ethyl 1H-pyrrole-2-carbonothioylcarbamate;NSC247000;Maybridge4_002728;MLS000702391;CHEMBL1577380;DTXSID90353375;HMS1528L22;HMS2604A20;NSC 247000;NSC-247000;IDI1_032606;N-ethoxycarbonylpyrrole-2-thiocarboxamide;SMR000226376;Ethyl (1H-pyrrole-2-carbothioyl)carbamate;AG-777/36177051