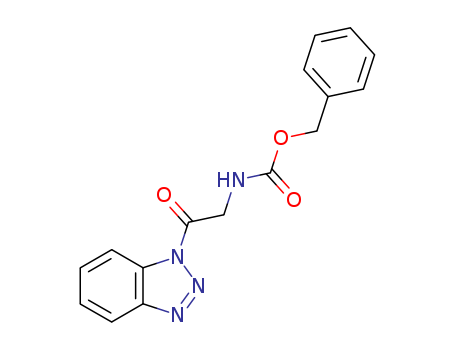
- Chemical Name:Benzyl 2-(1H-benzo[d][1,2,3]triazol-1-yl)-2-oxoethylcarbamate
- CAS No.:173459-80-8
- Molecular Formula:C16H14N4O3
- Molecular Weight:310.312
- Hs Code.:
- Mol file:173459-80-8.mol
Synonyms:benzyl [2?(1H?benzo[d][1,2,3]triazol?1?yl)?2?oxoethyl]carbamate