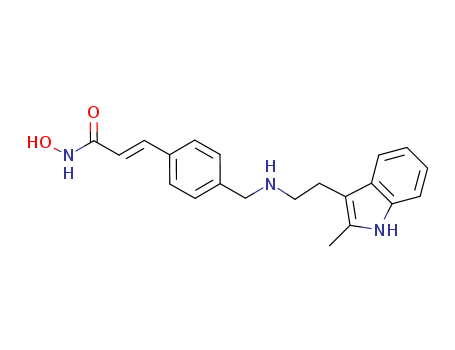
- Chemical Name:Panobinostat
- CAS No.:404950-80-7
- Molecular Formula:C21H23N3O2
- Molecular Weight:349.433
- Hs Code.:29339900
- European Community (EC) Number:803-814-1
- UNII:9647FM7Y3Z
- DSSTox Substance ID:DTXSID40193506
- Nikkaji Number:J2.554.199E,J3.451.397J
- Wikipedia:Panobinostat
- Wikidata:Q7131441
- NCI Thesaurus Code:C66948
- RXCUI:1603350
- Pharos Ligand ID:DV83J283Y51H
- Metabolomics Workbench ID:149853
- ChEMBL ID:CHEMBL483254
- Mol file:404950-80-7.mol
Synonyms:Farydak;LBH 589;LBH589;NVP LBH589;NVP-LBH589;panobinostat