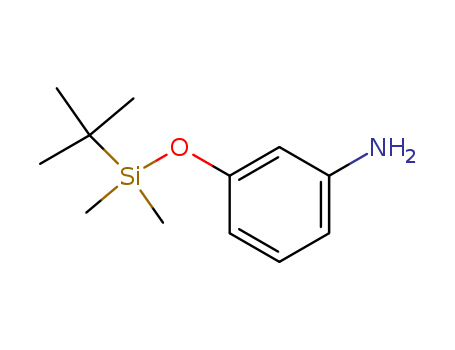
- Chemical Name:Benzenamine, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-
- CAS No.:121942-75-4
- Molecular Formula:C12H21NOSi
- Molecular Weight:223.39
- Hs Code.:
- European Community (EC) Number:860-045-4
- DSSTox Substance ID:DTXSID20558795
- Nikkaji Number:J1.894.855I
- Wikidata:Q82441221
- Mol file:121942-75-4.mol
Synonyms:121942-75-4;3-((tert-Butyldimethylsilyl)oxy)aniline;Benzenamine, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-;3-[tert-butyl(dimethyl)silyl]oxyaniline;3-(tert-butyldimethylsilyloxy)aniline;3-[(tert-Butyldimethylsilyl)oxy]aniline;3-aminophenol, TBDMS;SCHEMBL356737;DTXSID20558795;3-(tert-butyl dimethylsilyloxy)phenylamine;3-(tert-butyldimethylsilanyloxy)phenylamine;3-{[tert-Butyl(dimethyl)silyl]oxy}aniline;3-(tert-butyl dimethylsilyloxy) phenylamine;CS-0020567;3-(tert-Butyl-dimethyl-silanyloxy)-phenylamine;D72534;EN300-7461715;A1-19879