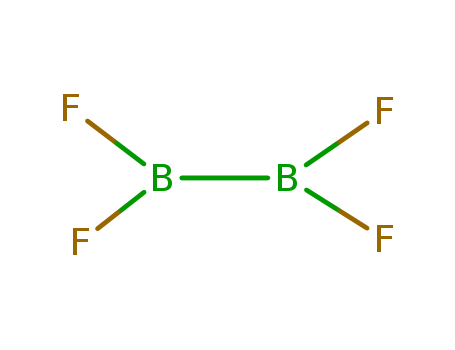
- Chemical Name:Diboron tetrafluoride
- CAS No.:13965-73-6
- Molecular Formula:B2F4
- Molecular Weight:97.6156
- Hs Code.:
- DSSTox Substance ID:DTXSID80161143
- Nikkaji Number:J1.411.353C
- Wikipedia:Diboron tetrafluoride
- Wikidata:Q2183990
- Mol file:13965-73-6.mol
Synonyms:Diboron tetrafluoride;13965-73-6;difluoroboranyl(difluoro)borane;difluoroboranyl-difluoro-borane;B2F4;Tetrafluorodiborane(4);Diborane(4), tetrafluoro-;DTXSID80161143;FT-0777907;Q2183990