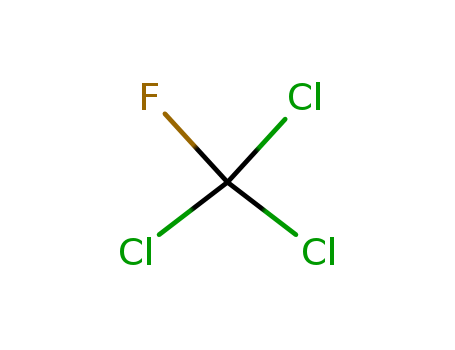
- Chemical Name:Trichlorofluoromethane
- CAS No.:75-69-4
- Deprecated CAS:62185-70-0,79620-41-0,83589-40-6,91315-61-6,79620-41-0,91315-61-6
- Molecular Formula:CCl3F
- Molecular Weight:137.368
- Hs Code.:2903771000
- European Community (EC) Number:200-892-3
- ICSC Number:0047
- UN Number:1956,3082
- UNII:990TYB331R
- DSSTox Substance ID:DTXSID5021384
- Nikkaji Number:J1.461C
- Wikipedia:Trichlorofluoromethane
- Wikidata:Q423000
- NCI Thesaurus Code:C76755
- Metabolomics Workbench ID:56915
- ChEMBL ID:CHEMBL348290
- Mol file:75-69-4.mol
Synonyms:FC-11;fluorotrichloromethane;Freon 11;Genetron 11;trichlorofluoromethane;trichlorofluoromethane hydrate;trichlorofluoromethane, (18)F-labeled;trichloromonofluoromethane


 Xn;
Xn;  N
N
 Xn:Harmful;
Xn:Harmful;

