Identification
CAS: 151454-11-4
- Name:
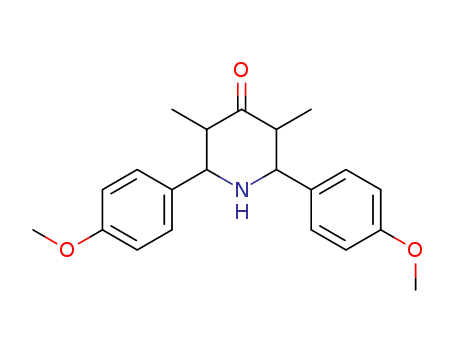
- 4-Piperidinone, 2,6-bis(4-methoxyphenyl)-3,5-dimethyl-
- Cas No.:
- 151454-11-4
- Molecular Structure:
-
- EINECS(EC#):
- Molecular Formula:
- C21H25NO3
- Molecular Weight:
- 339.434
- Synonyms:
151454-11-4 Chemical
- Appearance:
- mp :
- bp :
- Flash Point:
- Density:
- Water Solubility :
151454-11-4 Safety Data
Risk Codes:
Safety:
RTECS :
DG0875000
Well-known Company Product Price
| Brand | (Code)Product description | CAS number | Packaging | Price | Detail |
|---|
Post a RFQ
Prodcuts Directory
Suppliers list
- Henan Tianfu Chemical Co., Ltd.
- Telephone: 86-371-55170693/55170694
- Contact Fax: 86-371-55170690
- Website:http://www.tianfuchem.com/
- ZHEJIANG JIUZHOU CHEM CO.,LTD
- Telephone: +86 19334956669
- Contact Fax:
- Website:http://www.jiuzhou-chem.com
- Chemical Technology Co.,LTD
- Telephone:
- Contact Fax:
- Website:
- ENAO Chemical Co, Limited
- Telephone: 86--15307605669
- Contact Fax: 86--
- Website:www.enaochem.com
