127045-41-4 Usage
Description
Different sources of media describe the Description of 127045-41-4 differently. You can refer to the following data:
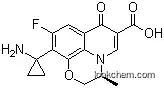
1. Pazufloxacin is a fluoroquinolone with a 1-aminocyclopropyl substituent at C10 position. The presence of aminoacyl group at C-10 is a unique feature of the molecule imparting potent broad spectrum activity against gram-positive and gram-negative bacteria. This activity is based on the inhibition of bacterial DNA gyrase.
Pazufloxacin is used as an injectable antibiotic with bacterial effect against cephalosporin-resistant, carbapenem-resistance, and aminoglycoside resistant strains of bacteria. The adverse effects of pazufloxacin, such as drug-induced convulsion and hypotension are less than those of other conventional injectable fluoroquinolones.
2. Pazufloxacin is a novel quinolone marketed for the treatment of bacterial infections in
Japan. This tricyclic fluoro-quinolone can be synthesized in 11 steps from commercially
available 2,3,4,5tetrafluorobenzoic acid. The cyclopropyl substituent is first introduced in 6
steps including 4-F-substitution with tert-butylcyanoacetate, decarboxylation, aa alkylation
with 1 ,Zdibromoethane, partial nitrile hydrolysis and Hoffmann-rearrangement. The
pyridoxazine ring is then introduced in 5 steps including 6-ketoester formation and
pryridoxazine annulation. Pazufloxacin displays a broad spectrum activity against Grampositive
and Gram-negative bacteria, although it is less active that ciprofloxacin against
pneumococci and is not active against ciprofloxacin-resistant isolates. In patients with
gonococcal urethritis a high prevalence of fluoroquinolone-resistant N. gonorrhoeae
isolates with the Ser-91-to-Phe mutation in GyrA was observed. However, good clinical
responses have been seen in clinical trials of patients with urinary tract infections and to a
lesser extent with respiratory tract infections. Pazufloxacin is mainly excreted in urine with
a short half-life (2-2.5 h). It has a phototoxicity equal to that of ciprofloxacin and its adverse
effect profile resembles that of other quinolones.
References
[1] A. Vora, Pazufloxacin, tracked from www.japi.org on 16.07.2017
[2] Jeffrey K. Aronson, Meyler’s Side Effects of Antimicrobial Drugs, 2009
[3] Satoshi Watabe, Yoshiaki Yokoyama, Kazuyuki Nakazawa, Kimikazu Shinozaki, Rika Hiraoka, Kei Takeshita and Yukio Suzuki, Simultaneous measurement of pazufloxacin, ciprofloxacin, and levofloxacin in human serum by high-performance liquid chromatography with fluorescence detection, Journal of Chromatography B, 2010, vol. 878, 1555-1561
Originator
Toyama (Japan)
Uses
Different sources of media describe the Uses of 127045-41-4 differently. You can refer to the following data:
1. Pazufloxacin is a potential antimicrobial and/or antiviral agent.
2. antibactierial
Brand name
Pasil, Pazucross
Pharmaceutical Applications
A tricyclic fluoroquinolone, formulated as mesylate and hydrochloride salts for oral or parenteral use or as a methane sulfonate (eye ointment).It displays good activity in vitro against methicillin
susceptible Staph. aureus (MIC 0.2 mg/L), but is inactive against Str. pyogenes, Str. pneumoniae (MIC ≥4 mg/L) and enterococci. L. pneumophila is inhibited by 0.03 mg/L. Activity against Enterobacteriaceae, fastidious Gram-negative bacilli, Ps. aeruginosa and Acinetobacter spp. is similar to that of ofloxacin. It is weakly active against Sten. maltophilia and Burkholderia cepacia (MIC c. 2 mg/L). Against M. tuberculosis, MICs range from 0.8 to 4 mg/L. It is inactive against anaerobes.After oral doses of 100 or 400 mg, peak plasma concentrations range from 0.94 mg/L (100 mg) to 4.5 mg/L (400 mg) after <1 h. The apparent elimination half-life is around 2 h. Most of the administered dose is eliminated in urine, about 70% within 24 h. Four metabolites have been reported. In elderly patients, according to the renal function, the peak plasma concentration may be elevated (up to 5.6 mg/L) and significantly delayed (2–6 h). The plasma protein binding ranges from 17% to 28%.
Check Digit Verification of cas no
The CAS Registry Mumber 127045-41-4 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 1,2,7,0,4 and 5 respectively; the second part has 2 digits, 4 and 1 respectively.
Calculate Digit Verification of CAS Registry Number 127045-41:
(8*1)+(7*2)+(6*7)+(5*0)+(4*4)+(3*5)+(2*4)+(1*1)=104
104 % 10 = 4
So 127045-41-4 is a valid CAS Registry Number.
InChI:InChI=1/C16H15FN2O4/c1-7-6-23-14-11(16(18)2-3-16)10(17)4-8-12(14)19(7)5-9(13(8)20)15(21)22/h4-5,7H,2-3,6,18H2,1H3,(H,21,22)
127045-41-4Relevant articles and documents
Pyridonecarboxylic acids as antibacterial agents. IX. Synthesis and structure-activity relationship of 3-substituted 10-(1-aminocyclopropyl)-9-fluoro-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de] -1,4-benzoxazine-6-carboxylic acids and their 1-thio and 1-aza anal
Todo,Takagi,Iino,Fukuoka,Takahata,Okamoto,Saikawa,Narita
, p. 2569 - 2574 (2007/10/02)
A series of the title compounds listed in Chart 1 have been synthesized to study the effects of 3-alkyl substituents on the antibacterial potency and in vivo efficacy of 10-(1-aminocyclopropyl)-9-fluoro-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de] -1,4-benzoxazi