- Buprenorphine
- Buprenorphine hydrochloride
- 52486-19-81-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione
- 52488-28-5Benzene,1-bromo-3-methyl-5-nitro-
- 52488-36-54-Bromoindole
- 52490-15-0Methanone,(2-butyl-3-benzofuranyl)(4-hydroxyphenyl)-
- 52-49-31-Piperidinepropanol, a-cyclohexyl-a-phenyl-, hydrochloride (1:1)
- 52494-32-35-[(3-Methylphenyl)amino]-3H-1,3,4-thiadiazole-2-thione
- 52494-33-41,3,4-Thiadiazole-2(3H)-thione,5-[(2-methoxyphenyl)amino]-
- 52494-50-5Hydrazinecarbothioamide,2,2'-(1,4-phthalazinediyl)bis[N-methyl-
- 52494-53-81,2,4-Triazolo[3,4-a]phthalazine,6-chloro-
- 52495-41-7Aurate(1-),tetrabromo-, sodium (1:1), (SP-4-1)-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
| Name |
Buprenorphine |
EINECS | 257-950-6 |
| CAS No. | 52485-79-7 | Density | 1.26g/cm3 |
| PSA | 62.16000 | LogP | 5.15370 |
| Solubility | N/A | Melting Point |
260-262°C |
| Formula | C29H41 N O4 | Boiling Point | °Cat760mmHg |
| Molecular Weight | 467.649 | Flash Point | °C |
| Transport Information | N/A | Appearance | N/A |
| Safety | Poison by ingestion, intravenous, and intraperitoneal routes. Note: This is a controlled substance (narcotic) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.15 (1985). When heated to decomposition it emits toxic fumes of NOx. | Risk Codes | 11-23/24/25-39/23/24/25 |
| Molecular Structure |
|
Hazard Symbols | F,T |
| Synonyms |
6,14-Ethenomorphinan-7-methanol,17-(cyclopropylmethyl)-a-(1,1-dimethylethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-a-methyl-, [5a,7a(S)]-; Buprenorfine; Buprenorphine; Norspan; Norvex;RX 6029M; Subutex; Temgesic; Transtec |
Article Data | 24 |
Buprenorphine Chemical Properties
Molecular Structure of Buprenorphine (CAS NO.52485-79-7):
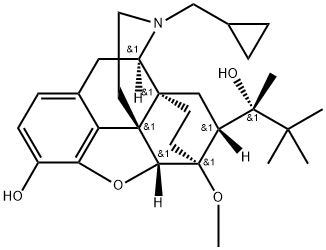
EINECS: 257-950-6
Molecular Formula: C29H41NO4
Molecular Weight: 467.640140 g/mol
XLogP3: 5
H-Bond Donor: 2
H-Bond Acceptor: 5
Isomeric SMILES: CC(C)(C)C(C)([C@H]1C[C@@]23CC[C@]1(C4[C@@]25CCN([C@@H]3CC6=C5C(=C(C=C6)O)O4)CC7CC7)OC)O
InChI: InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24,26,27-,28+,29+/m1/s1
InChIKey: RMRJXGBAOAMLHD-BHKLSUIZSA-N
Index of Refraction: 1.632
Molar Refractivity: 131.37 cm3
Molar Volume: 368.2 cm3
Surface Tension: 58.2 dyne/cm
Density: 1.26 g/cm3
Melting Point: 260-262 °C
Water Solubility: 0.6554 mg/L at 25 °C
Buprenorphine History
Buprenorphinewith CAS registry number of 52485-79-7 was first marketed in the 1980s by the pharmaceutical company, Reckitt Benckiser as Temgesic 0.2 mg sublingual tablets, and as Buprenex in a 0.3 mg/ml injectable formulation. Suboxone and Subutex as sublingual pill preparations for opioid addiction treatment was approved by FDA in October 2002 and was approved in the European Union in September 2006. In recent years, buprenorphine has been introduced in most European countries as a transdermal formulation for the treatment of chronic pain.
Buprenorphine Uses
Buprenorphine with cas registry number of 52485-79-7 is used for the treatment of moderate to severe chronic pain or for peri-operative analgesia. The transdermal formulations is used in chronic cancer pain as well as chronic non-malignant pain, the intravenous formulation is mainly used in postoperative pain, and the sublingual formulation is used as breakthrough medication for patients with basic transdermal treatment. Advantages are its relatively long half-life, the option of sublingual and transdermal application and the excellent safety profile. Buprenorphine is also used recreationally by opioid users. Buprenorphine abuse is very common in Scandinavia, especially in Finland and Sweden. In addition, this product can be used for treatment of opiate addiction first reported in the United States by Dr. David McDowell at Columbia University.
Buprenorphine Toxicity Data With Reference
| 1. | ipr-rat LD50:197 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 17 (1979),81. | ||
| 2. | ivn-rat LD50:31 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 17 (1979),81. | ||
| 3. | orl-mus LD50:260 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 17 (1979),81. | ||
| 4. | ipr-mus LD50:90 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 17 (1979),81. | ||
| 5. | ivn-mus LD50:24 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 17 (1979),81. |
Buprenorphine Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Note: This is a controlled substance (narcotic) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.15 (1985). When heated to decomposition it emits toxic fumes of NOx.
Buprenorphine Specification
Buprenorphine with CAS registry number of 52485-79-7 is white solid, known as 17-Cyclopropylmethyl-4,5alpha-epoxy-7alpha-((S)-1-hydroxy-1,2,2-trimethylpropyl-6-methoxy-6,14-endo-ethanomorphinan-3-ol ; 2-(N-Cyclopropylmethyl-4,5alpha-epoxy-3-hydroxy-6-methoxy-6,14-endo-ethanomorphinan-7alpha-yl)-3,3-dimethyl-2-butanol ; 21-(Cyclopropyl-7alpha-((S)-1-hydroxy-1,2,2-trimethylpropyl-6,14-endo-ethano-6,7,8,14-tetrahydrooripavine ; 6029-M ; Buprenorfina ; Buprenorfina [INN-Spanish] ; Buprenorphine [INN:BAN] ; Buprenorphinum ; Buprenorphinum [INN-Latin] ; Buprenex ; DEA No. 9064 ; Probuphenine ; Temgesic ; UNII-40D3SCR4GZ ; 6,14-Ethenomorphinan-7-methanol, 17-(cyclopropylmethyl)-alpha-(1,1-dimethylethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-alpha-methyl-, (5-alpha,7-alpha-(S))- . In market, it is sold under names of Suboxone and Subutex with partial agonist actions at the mu opioid receptor and antagonist actions at other opioid receptors. In the Netherlands, Buprenorphine is a List II drug of the Opium Law, though special rules and guidelines apply to its prescription and dispensation. In the USA, it has been a Schedule III drug under the United Nations' Convention on Psychotropic Substances since it was rescheduled from Schedule V just before FDA approval of Suboxone and Subutex.