- 9003-36-5FORMALDEHYDE, POLYMER WITH (CHLOROMETHYL)OXIRANE AND PHENOL
- 9003-39-8Povidone
- 9003-49-02-Propenoic acid, butylester, homopolymer
- 90035-34-0[1,1'-Biphenyl]-4-carboxaldehyde,4'-(trifluoromethyl)-
- 9003-53-6Poly(styrene)
- 9003-54-7Poly(styrene-co-acrylonitrile)
- 9003-55-8Benzene, ethenyl-, polymer with 1,3-butadiene
- 9003-56-9ABS Resins
- 9003-59-2POLY(SODIUM 4-STYRENESULFONATE)
- 9003-74-1Terpenes, polymers
Hot Products
- 60643-86-9Vigabatrin
- 36290-04-7Formaldehyde-2-naphthalenesulfonic acid copolymer sodium salt
- 10124-48-8Aminomercuric chloride
- 544-31-0Hexadecanamide,N-(2-hydroxyethyl)-
- 174649-09-32-Oxazolidinone, 3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-[[(methylsulfonyl)oxy]methyl]-, (5R)-
- 71550-12-4Poly(allylamine hydrochloride)
- 1310-53-8Germanium oxide (GeO2)
- 1317-35-7Manganese oxide (Mn3O4)
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Phenol-formaldehyde resin |
EINECS | 1533716-785-6 |
| CAS No. | 9003-35-4 | Density | 1.10 g/cm3 |
| PSA | 37.30000 | LogP | 1.84320 |
| Solubility | 1.557mg/L at 25℃ | Melting Point |
94 °C |
| Formula | (C6H6O.CH2O)x | Boiling Point | 229.3℃[at 101 325 Pa] |
| Molecular Weight | 124.13700 | Flash Point | N/A |
| Transport Information | N/A | Appearance | light yellow granule |
| Safety | Risk Codes | N/A | |
| Molecular Structure |
|
Hazard Symbols | N/A |
| Synonyms |
Formaldehyde phenol polymer;Paraformaldehyde, formaldehyde, phenol polymer;Paraformaldehyde, phenol polymer;Phenol-formaldehyde resin;Phenol-formaldehyde resin;Phenolic moulding powder; |
Article Data | 2 |
Phenol-formaldehyde resin History
Phenolic resins(9003-35-4), copolymers of phenol and formaldehyde, were the first fully synthetic polymers made. They were discovered in 1910 by Leo Baekeland and given the trade name Bakelite.
Phenol-formaldehyde resin Specification
The Phenol-formaldehyde resin, with the CAS registry number 9003-35-4,is also known as 2-Phenylethylamine; 3-Aminoethylbenzene. It belongs to the product categories of Polymers. This chemical's molecular formula is (C6H6O)n.(CH2O)n.What's more,Its systematic name is Phenol,polymer with formaldehyde.It is Stable,incompatible with strong oxidizing agents.It can be used to make molded products including pool balls, laboratory countertops, and as coatings and adhesives.
Production of Phenol-formaldehyde resin:
Two processes, both involving step growth polymerization, are used for the manufacture of phenolic resins.
A one-stage resin may be obtained by using an alkaline catalyst and excess formaldehyde to form linear, low-molecular-weight resol resins. Acidification and further heating causes the curing process to give a highly cross-linked thermoset polymer. The o- and p-ethylolphenols are more reactive toward formaldehyde than the original phenol and rapidly undergo further reaction to give di- and trimethylol derivatives. The methylolphenols will react to form di- and trinuclear phenols at still-free ortho and para positions. The final structure of the product involves a high degree of branching. Most linkages between aromatic rings are methylone (CH2) groups, though some other (CH2.OCH2) linkages are present.
The second process (Fig. l) uses an acid catalyst and excess phenol to give a linear polymer (novolac) that has no free methylol groups for cross-linking. In a separate second part of this two-stage process, a cross-linking agent is added and further reaction occurs. In many instances, hexamethylenetetramine is used, which decomposes to formaldehyde and ammonia.
Other modifications in making phenolic polymers are the incorporation of cresols or resorcinol as the phenol (Fig. 1) and acetaldehyde or furfural as the phenol.
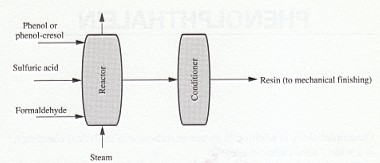
FIGURE 1 Manufacture of phenolic resins.
The toxicity data of Phenol-formaldehyde resin are as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | National Technical Information Service. Vol. OTS0556084, | |
| rat | LD50 | skin | > 2gm/kg (2000mg/kg) | National Technical Information Service. Vol. OTS0556084, |

