- Ammonium (-)-3-bromo-8-camphorsulfonate
- Ammonium (S)-5-((4-amino-4-carboxy-1-oxobutyl)amino)-2-nitrobenzoate
- Ammonium 1-pyrrolidinedithiocarbamate
- Ammonium 2,4-dichlorophenoxyacetate
- Ammonium 2-hydroxyethanesulphonate
- Ammonium 4-nitrobenzoate dihydrate
- Ammonium acetate
- Ammonium adipate
- Ammonium alginate
- Ammonium azide
- 10029-00-2Ethanone,1-tricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaen-5-yl-
- 10029-04-6Ethyl 2-(hydroxymethyl)prop-2-enoate
- 10029-09-11,4-Cyclohexanedimethanamine,cis-
- 100296-71-7Bis(2-(2(or4)-isododecylphenoxy)ethyl) hydrogen phosphate, compound with 2,2,2-nitrilotris(ethanol)
- 100-29-8Benzene,1-ethoxy-4-nitro-
- 100299-04-5Willow, Salix caspica,ext.
- 100299-08-94H-Pyrido[1,2-a]pyrimidin-4-one,9-methyl-3-(2H-tetrazol-5-yl)-, potassium salt (1:1)
- 100303-57-9Oxirane,2-[[3-(methoxydimethylsilyl)propoxy]methyl]-
- 1003-03-8Cyclopentanamine
- 1003043-46-6Boronic acid, B-(2,6-diethoxy-3-pyridinyl)-
Hot Products
- 116-14-3Ethene,1,1,2,2-tetrafluoro-
- 14314-42-2Water-18O
- 175357-18-3L-Phenylalanine,N-(1-oxo-10-undecen-1-yl)-
- 25596-24-1Sulfoxonium,trimethyl-, bromide (8CI,9CI)
- 68855-54-9Kieselguhr, soda ash flux-calcined
- 25583-20-4Titanium nitride (TiN)
- 68441-17-8Ethene, homopolymer, oxidized
- 57-97-6Benz[a]anthracene,7,12-dimethyl-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

| Name |
Ammonium stearate |
EINECS | 213-695-2 |
| CAS No. | 1002-89-7 | Density | 0.8900 |
| PSA | 40.13000 | LogP | 5.37400 |
| Solubility | slightly soluble in Water | Melting Point |
21-24℃ |
| Formula | C18H36 O2 . H3 N | Boiling Point | 359.4 °C at 760 mmHg |
| Molecular Weight | 301.5078 | Flash Point | 162.4 °C |
| Transport Information | N/A | Appearance | cream or light yellow powder |
| Safety | A nuisance dust. When heated to decomposition it emits toxic vapors of NH3. | Risk Codes | 36/37/38 |
| Molecular Structure |
|
Hazard Symbols | A nuisance dust. TLV: TWA 10 mg/m3. |
| Synonyms |
Octadecanoicacid, ammonium salt (9CI); Stearic acid, ammonium salt (8CI); Ammoniumstearate; DC 100A; Kanebinol YC 81; Ligafluid AS 35; Nopco DC 100A; Nopcote DC100A; Stanfax 320; Stokal STA; YC 81 |
Ammonium stearate Chemical Properties
Molecular Structure:
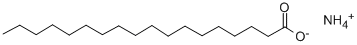
Molecular Formula: C18H39NO2
Molecular Weight: 301.5078
IUPAC Name: Azanium octadecanoate
Synonyms of Ammonium stearate (CAS NO.1002-89-7): AI3-03102 ; EINECS 213-695-2 ; Octadecanoic acid, ammonium salt ; Stearates ; Ammonium stearate, pure ; Octadecanoic acid, ammonium salt (1:1) ; Stearic acid, ammonium salt
CAS NO: 1002-89-7
Stearic acid, ammonium salt
Stability: Incompatible with strong oxidizing agents
Apperance: White to yellow paste or liquid with a weak ammonia odor
Flash Point: 162.4 °C
Enthalpy of Vaporization: 63.84 kJ/mol
Boiling Point: 359.4 °C at 760 mmHg
Vapour Pressure: 8.58E-06 mmHg at 25°C
Ammonium stearate Uses
Ammonium stearate (CAS NO.1002-89-7) is used as emulsifier, dispersant, wetting agent, moisture etc.
Ammonium stearate Consensus Reports
Reported in EPA TSCA Inventory.
Ammonium stearate Safety Profile
A nuisance dust. When heated to decomposition it emits toxic vapors of NH3.
Ammonium stearate Standards and Recommendations
ACGIH TLV: TWA 10 mg/m3
Ammonium stearate Specification
Ammonium stearate (CAS NO.1002-89-7) has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.

