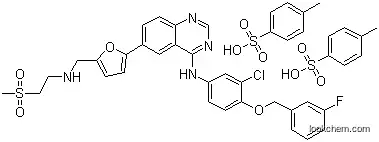
388082-77-7Relevant articles and documents
An environment friendly process for the preparation of Lapatinib Ditosylate of Formula 1(b)
-
, (2022/02/15)
The present invention relates to a process for the preparation of Lapatinib Ditosylate of formula 1(b). More particularly, the present invention relates to environment friendly process that involves green chemistry in preparation of Lapatinib Ditosylate of formula 1(b). The said process is economically and commercially viable as initial 2 stages of processes use water as solvent avoiding hazardous reagent or solvent during the preparation of Lapatinib Ditosylate of formula 1(b).
IMPROVED PROCESS FOR THE PREPARATION OF LAPATINIB BASE AND IT'S ANHYDROUS DITOSYLATE SALT
-
Paragraph 11, (2020/07/15)
The present invention relates to an improved, high yielding and industrially viable process for the preparation of high pure Lapatinib of formula (1). The present invention involves simple crystallization techniques avoiding column chromatographic techniques and the process conditions can be easily adopted for scale-up studies.
A high-purity paratoluene sulfonic acid lapatinib a preparation method of water composition
-
Paragraph 0020, (2019/04/04)
The invention relates to a high-purity paratoluene sulfonic acid lapatinib a water composition of the preparation method, the method comprises the following steps: (1) the compound II with compound III under alkaline conditions, the catalyst under the action of the Suzuki coupling to obtain compound IV; (2) the compound IV with a compound V in weakly alkaline conditions, in the catalyst under the action of the joint, by the three-b acyl sodium borohydride reduction, paratoluene sulfonic acid to form the salt to obtain compound VI; (3) the compound VI with the recrystallization to obtain compound I, is the second-to-toluene sulfonic acid lapatinib a hydrate. The programme provides at least to a certain extent one of the solve the above technical problems or at least provide a useful commercial choice. To avoid the use of toxicity is relatively high, the environmentally harmful solvent or reagent, the reaction route is operating time is short, the reaction system is stable, the purification process is simple, the product purity is higher, it is suitable for industrial production.
Method for synthesizing lapatinib or intermediate 5-(4-hydroxy quinazoline)-furan-2-formaldehyde of lapatinib
-
Paragraph 0034; 0051; 0052; 0053, (2017/03/22)
The invention relates to a method for synthesizing lapatinib or intermediate 5-(4-hydroxy quinazoline)-furan-2-formaldehyde of lapatinib. The method for synthesizing the intermediate comprises steps as follows: 4-hydroxy-6-nitro quinazoline reacts with hydrazine hydrate in a solvent in the presence of a catalytic amount of catalyst, and 4-hydroxy-6-amino quinazoline is prepared; 4-hydroxy-6-amino quinazoline reacts with furaldehyde in the solvent in presence of acid, sodium nitrite and a catalytic amount of catalyst, and the intermediate is prepared. The invention further relates to a method for synthesizing lapatinib and/or a lapatinib salt, a lapatinib intermediate and/or a pharmaceutically acceptable salt of the intermediate. The method is performed by using the intermediate synthesized with the method. The method has the advantages that steps are simplified, agents are cheap, available and high in utilization rate, heavy metal pollution is avoided, the reaction condition/operation requirement is lower and/or the yield is high and the like.
Method for synthesizing lapatinib or intermediate thereof
-
, (2017/06/30)
The invention relates to a method for synthesizing lapatinib or an intermediate thereof. The method for synthesizing the intermediate comprises the steps as follows: under the condition that a catalytic quantity of a catalyst exists in a solvent, 4-amino-5-(4-(3-chloro-4-(3-fluorobenzyloxy) phenylamino)-quinazoline reacts with furfural for preparation of 5-(4-(3-chloro-4-(3-fluorobenzyloxy) phenylamino)-quinazoline-6-yl)furyl-2-carboxaldehyde hydrochloride, and the intermediate is prepared. The invention further relates to a method for synthesizing lapatinib and/or salt of lapatinib, the intermediate of lapatinib and/or pharmaceutically acceptable salt of the intermediate, and the method is performed with the intermediate which is synthesized by the previous method. The method has the advantages that steps are simplified, a reagent is cheap, available and high in use ratio, pollution from heavy metal is avoided, and requirements for reaction conditions/operation are relatively low and/or the yield is high.
Preparing method of lapatinib and preparing method of lapatinib ditosylate
-
, (2016/10/10)
The invention provides a preparing method of lapatinib. The preparing method includes the steps that under nitrogen protection, a compound in the formula (IV) (please see the specification), 5-formyl-2-furanboronic acid, organic base or inorganic base, tetrahydrofuran and ethanol are mixed, the temperature is increased to 60-70 DEG C, stirring is carried out, a palladium catalyst is added, and a reaction is carried out under the temperature control condition; after the mixture is completely reacted, filtering, filter liquor cooling, water dripping, stirring, filtering and drying are carried out, the obtained product and 2-(methylsulfonyl) ethylamine hydrochloride are further reacted under the condition of existing of glacial acetic acid, then NaBH(OAC)3 is added for a reduction reaction, and lapatinib is obtained. The invention further provides a preparing method of lapatinib ditosylate. The preparing method includes the steps that a tetrahydrofuran solution of p-toluenesulfonic acid-monohydrate is dropwise added into the lapatinib or a solution obtained before concentrating, and the lapatinib ditosylate is obtained. The methods are convenient to operate, small in solvent dosage, low in production cost, small in environment pollution, high in product yield and purity and more suitable for industrial production.
Lapatinib process for the preparation of intermediates
-
Paragraph 0027; 0028, (2017/01/17)
The invention discloses a preparation method of a Tykerb intermediate. The preparation method of the Tykerb intermediate 1 comprises the following steps: (1) performing a nucleophilic substitute reaction on a compound as shown in a formula 3 and a compound as shown in a formula 4 under the effect of a catalyst in an organic solvent, wherein X is chlorine or bromine, M is sodium, potassium or zinc, and n is equal to 1 or 2; and (2) enabling a compound 2 obtained in the step (1) to react in a hydrogen chloride solution. According to the preparation method of the Tykerb intermediate 2, the nucleophilic substitute reaction is carried out on the compound as shown in the formula 3 and the compound as shown in the formula 4 under the effect of the catalyst in the organic solvent. The preparation method disclosed by the invention is simple to operate, the raw materials are cheap and easy to obtain, no environment pollution factor is caused, and the method is suitable for large-scale production in industry.
CO-CRYSTALS OF LAPATINIB MONOACID SALTS
-
Paragraph 00117; 00118; 00119; 0020; 00121; 00122, (2015/11/10)
The present invention refers to co-crystals of monoacid salts of the pharmaceutical active ingredient named Lapatinib and to processes for the preparation thereof and medical uses.
CANCER TREATMENT METHOD
-
Paragraph 0163-0164, (2016/03/12)
A method of treating cancer is described including administration of a 4-quinazolineamine and at least one other anti-neoplastic agent as well as a pharmaceutical combination including the 4-quinazolineamines.
EFFICIENT PROCESS FOR THE PREPARATION OF LAPATINIB AND SALTS THEREOF BY MEANS OF NEW INTERMEDIATES
-
, (2015/03/16)
The present invention refers to a new efficient process for the synthesis of the active pharmaceutical ingredient Lapatinib and salts thereof. In particular, the present synthesis is carried out employing new intermediates in which the amine function is protected by a group cleavable in basic milieu that provides a higher overall yield of the synthesis process.