- Entecavir
- Entecavir hydrate
- 20921-96-4Benzeneacetic acid, 2-cyano-, methyl ester
- 20922-03-6Isoquinoline,1-(1-methylethyl)-
- 20924-05-41(2H)-Pyrimidineaceticacid, 3,4-dihydro-5-methyl-2,4-dioxo-
- 20924-56-51H-2-Benzopyran-4(3H)-one
- 2092-49-13,3-Biphenyldicarboxaldehyde, 6,6-dihydroxy-5,5-dimethoxy- (8CI)
- 209252-15-3Benzenepropanoic acid, b-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-,(bS)-
- 209252-16-4Benzenebutanoic acid, b-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-,(bR)-
- 209252-17-5Pentanedioic acid,3-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-, 1-(1,1-dimethylethyl) ester,(3R)-
- 20925-24-0Benzonitrile,2-(dimethylamino)-
- 20925-25-1Benzonitrile, 2-(1-pyrrolidinyl)-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Entecavir hydrate |
EINECS | 606-668-5 |
| CAS No. | 209216-23-9 | Density | 1.81 g/cm3 |
| PSA | 139.28000 | LogP | -0.31090 |
| Solubility | N/A | Melting Point |
>220° |
| Formula | C12H15N5O3.H2O | Boiling Point | 734.2 °C at 760 mmHg |
| Molecular Weight | 295.298 | Flash Point | 397.9 °C |
| Transport Information | N/A | Appearance | White crystalline powder |
| Safety | 24/25 | Risk Codes | N/A |
| Molecular Structure |
|
Hazard Symbols | N/A |
| Synonyms |
Baraclude;6H-Purin-6-one, 2-amino-1,9-dihydro-9-((1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl)-, monohydrate;Intermediates of drug, pesticide and dye;Enticavir;Entecavir hydrate / 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate;Entecavir monohydrate;Entecavir API in stock;Entecavir(monohydrate)--Fine Product; |
Article Data | 2 |
Entecavir hydrate Synthetic route

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In acetonitrile at 20℃; | 88% |

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In pyridine at 20℃; for 6h; |

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In pyridine at 20℃; for 6h; |

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In pyridine at 20℃; for 6h; |
- 209216-23-9
entecavir monohydrate

- 1383812-22-3
((1 R,3S,5S)-5-acetoxy-3-(2-amino-6-chloro-9H-purin-9-yl)-2-methylenecyclopentyl)methyl acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine; dmap / acetonitrile / 20 °C 2: N-benzyl-N,N,N-triethylammonium chloride; trichlorophosphate / acetonitrile; N,N-dimethyl-aniline / 1 h / 70 °C / Cooling with ice View Scheme |
- 209216-23-9
entecavir monohydrate

- 701278-58-2
6-chloro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylene-cyclopentyl]-9H-purine-2-amine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triethylamine; dmap / acetonitrile / 20 °C 2: N-benzyl-N,N,N-triethylammonium chloride; trichlorophosphate / acetonitrile; N,N-dimethyl-aniline / 1 h / 70 °C / Cooling with ice 3: ammonia / methanol / 5 h / 20 °C View Scheme |
- 209216-23-9
entecavir monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylamine; dmap / acetonitrile / 20 °C 2: N-benzyl-N,N,N-triethylammonium chloride; trichlorophosphate / acetonitrile; N,N-dimethyl-aniline / 1 h / 70 °C / Cooling with ice 3: ammonia / methanol / 5 h / 20 °C 4: zinc; ammonium hydroxide / 55 °C View Scheme |
Entecavir hydrate Chemical Properties
Molecular Structure of Entecavir hydrate (CAS NO.209216-23-9):
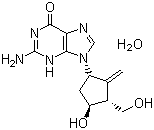
IUPAC Name: 2-amino-9-[(3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one hydrate
Molecular Formula: C12H15N5O3.H2O
Molecular Weight: 295.30
H bond acceptors: 9
H bond donors: 7
Freely Rotating Bonds: 4
Polar Surface Area: 125.76 Å2
Density:1.81g/cm3
Flash Point: 397.9 °C
Enthalpy of Vaporization: 112.46 kJ/mol
Boiling Point: 734.2 °C at 760 mmHg
Vapour Pressure: 1.24E-22 mmHg at 25°C
Appearance: White crystalline powder
Classification Code: Anti-Infective Agents; Antiviral Agents; Antiviral used in the treatment of hepatitis B infection; Treatment of hepatitis B infection
InChI
InChI=1/C12H15N5O3.H2O/c1-5-6(3-18)8(19)2-7(5)17-4-14-9-10(17)15-12(13)16-11(9)20;/h4,6-8,18-19H,1-3H2,(H3,13,15,16,20);1H2/t6-,7+,8-;/m0./s1
Smiles
[nH]1c(N)nc2[n@](cnc2c1=O)[C@H]1C([C@@H]([C@H](C1)O)CO)=C.O
Entecavir hydrate History
1992: SQ-34676 at Squibb as part of anti-herpes virus program.
1997: BMS 200475 developed at BMS pharmaceutical research institute as antiviral nucleoside analogue à Activity demonstrated against HBV, HSV-1, HCMV, VZV in cell lines & no or little activity against HIV or influenza.Superior activity observed against HBV pushed research towards BMS 200475, its base analogues and its enantiomer against HBV in HepG2.2.15 cell line Comparison to other NAs, proven more selective potent inhibitor of HBV by virtue of being Guanine NA.
1998: Inhibition of hepadnaviral polymerases was demonstrated in vitro in comparison to a number of NAs-TP. Metabolic studies showed more efficient phosphorylation to triphosphate active form.
3 year treatment of woodchuck model of CHB sustained antiviral efficacy and prolonged life spans without detectable emergence of resistance.
Entecavir hydrate Safety Profile
Entecavir hydrate (CAS NO.209216-23-9) may cause lactic acidosis and severe hepatomegaly with steatosis (including fatal cases) when used alone or in combination with antiretrovirals. Severe acute hepatitis B exacerbations have occurred in patients who discontinued anti-hepatitis B therapy, including entecavir. Monitor hepatic function for at least several months after discontinuation. If appropriate, initiate anti-hepatitis B therapy.
Entecavir hydrate Specification
Entecavir hydrate , with CAS number of 209216-23-9, can be called entecavir hydrate ; Entecavir monohydrate ; 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate ; Entecavir hydrate / 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate . Entecavir hydrate (CAS NO.209216-23-9) is an oral antiviral drug used in the treatment of hepatitis B infection.


