
Journal of Organometallic Chemistry p. 379 - 388 (1989)
Update date:2022-08-11
Topics:
 Bumagin, N. A.
Bumagin, N. A.
 More, P. G.
More, P. G.
 Beletskaya, I. P.
Beletskaya, I. P.
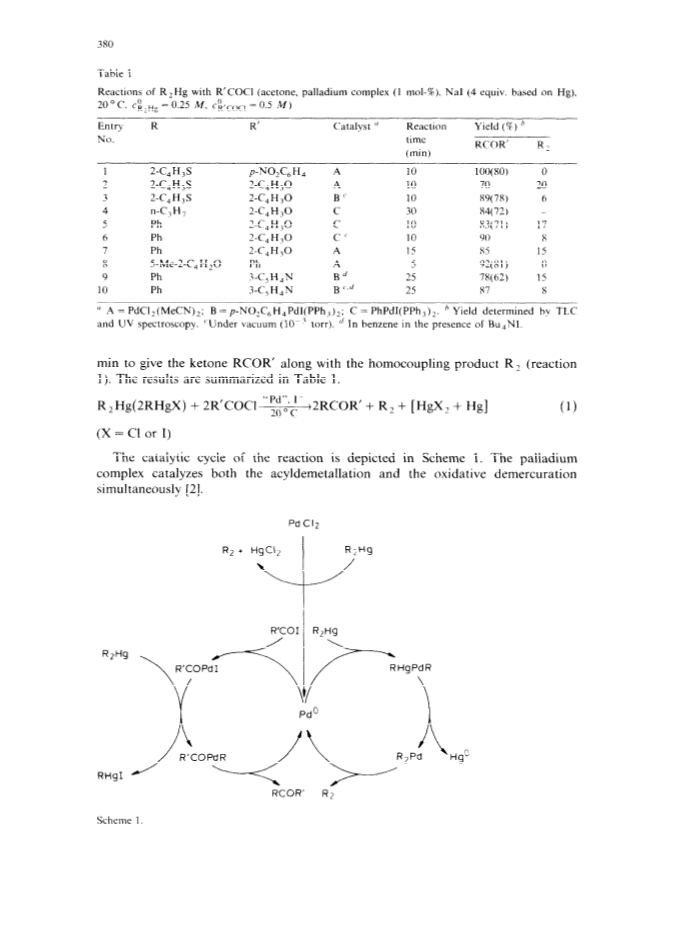
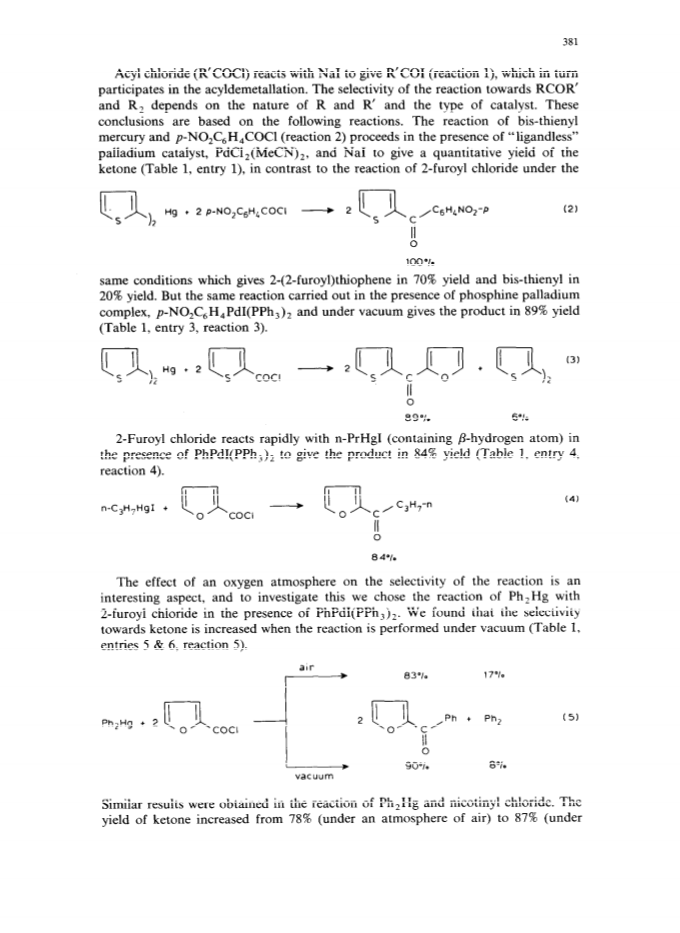
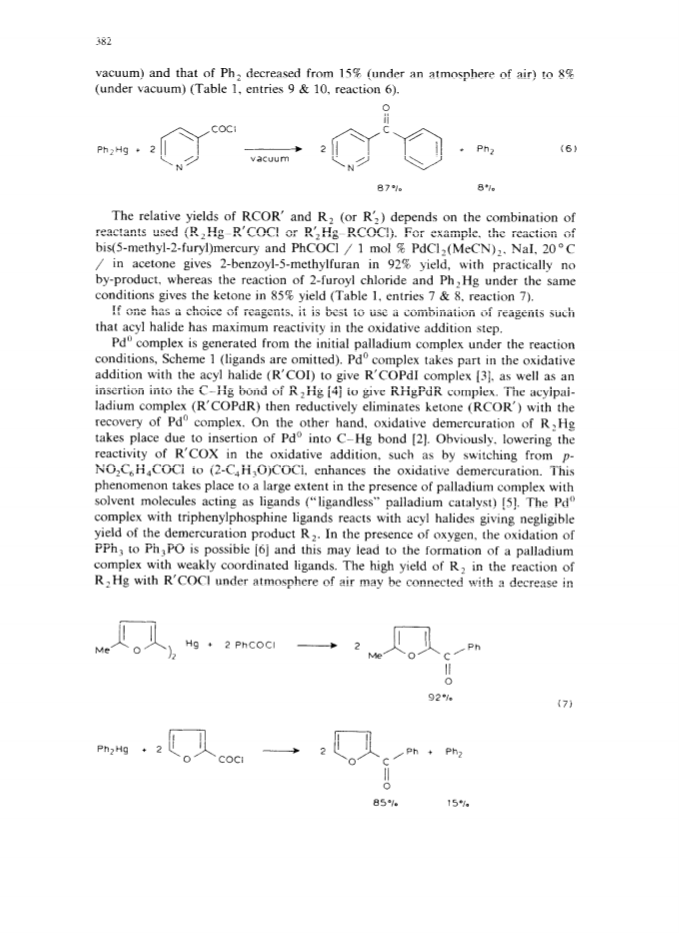
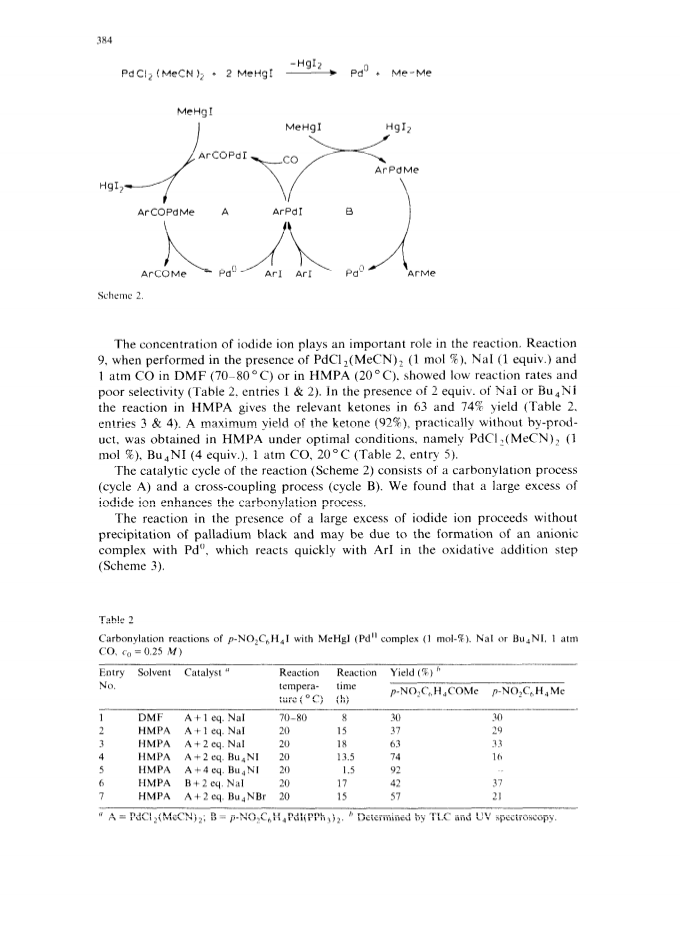
The palladium-catalyzed acyldemetallation reactions of organomercurials (R2Hg or AlkHgI) provide a mild, selective and general method for the synthesis of unsymmetrical heterocyclic ketones.High yields of ketones (RCOR') were obtained along with small amounts of homocoupling products (R2).The reaction is accelerated by a nucleophilic catalyst with both organic groups of R2Hg taking part in the reaction.The selectivity of the reaction towards the ketone can be increased by carrying out the reaction with a suitable combination of reactants under vacuum.The palladium-catalyzed carbonylation of organomercurials in the presence of ArI is another route to unsymmetrical ketones.The AlkHgI-ArI system undergoes carbonylation in the presence of palladium complex and excess iodide ion under mild conditions to give high yields of unsymmetrical ketones.The selectivity and rate of the reaction depend upon the nature of the catalyst, solvent and the concentration of iodide ion.
View More




website:http://www.joyochem.com
Contact:0531-82687558, 0531-82687998
Address:Factory Building 11, Jinan Comprehensive free trade zone, Shandong, China
Hangzhou Pharma & Chem Co.,Ltd.
Contact:+86-571-87040515
Address:No,139Qingchun Rd
Guangzhou PI & PI Biotech Inc. Ltd.
Contact:+86-20-81716320
Address:Suite 501,198 Kaiyuan Ave.,Science City, Guangzhou,China
Hebei Think-Do Environment Co., Ltd
website:http://www.thinkdo-environment.com
Contact:0311-86510809
Address:No 6, Shilian Middle Street, Circular Chemical Industry Park
Xian Changyue Biological Technology Co., Ltd.
website:https://www.xachangyue.com/
Contact:+86-029-62886900
Address:Keji Road NO.70
Doi:10.1039/jr9640003532
(1964)Doi:10.3184/030823407X218110
(2007)Doi:10.1039/c5ra19080k
(2015)Doi:10.1016/j.tetlet.2011.06.009
(2011)Doi:10.1016/j.dyepig.2018.04.034
(2018)Doi:10.1039/c39820000671
(1982)