
Dalton Transactions p. 14591 - 14602 (2016)
Update date:2022-08-17
Topics:
 Pretorius, René
Pretorius, René
 Fructos, Manuel R.
Fructos, Manuel R.
 Müller-Bunz, Helge
Müller-Bunz, Helge
 Gossage, Robert A.
Gossage, Robert A.
 Pérez, Pedro J.
Pérez, Pedro J.
 Albrecht, Martin
Albrecht, Martin
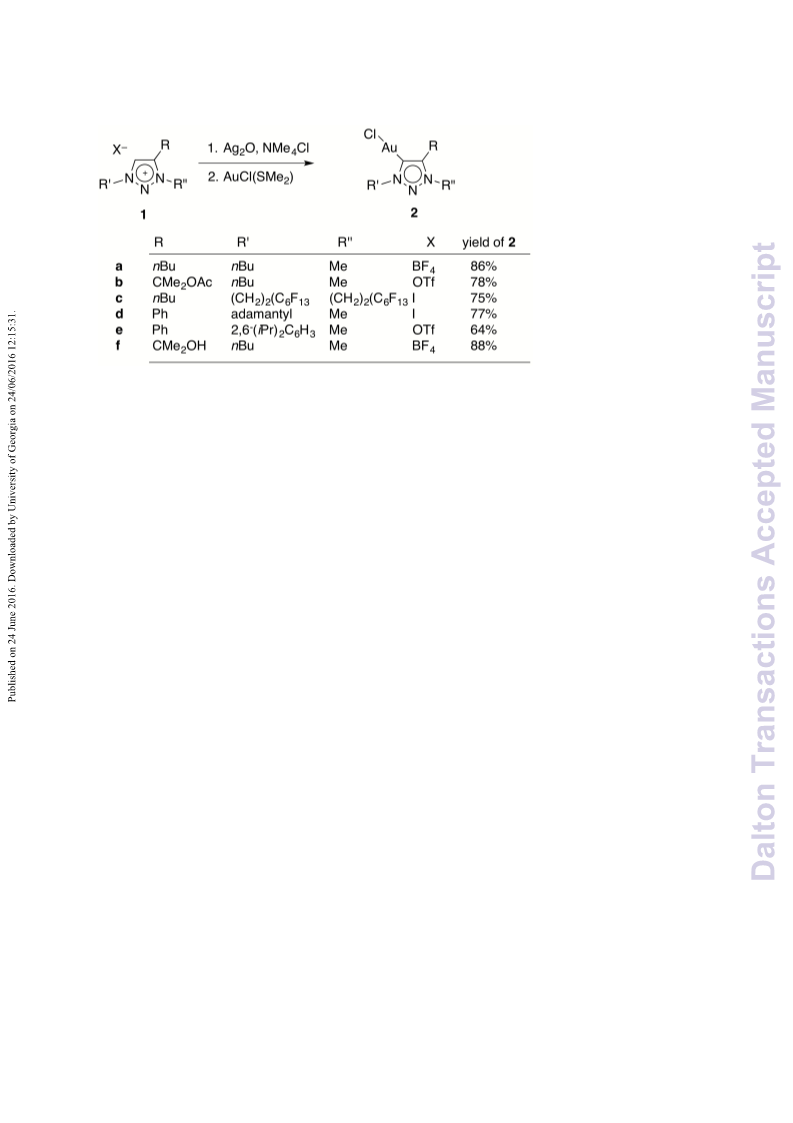
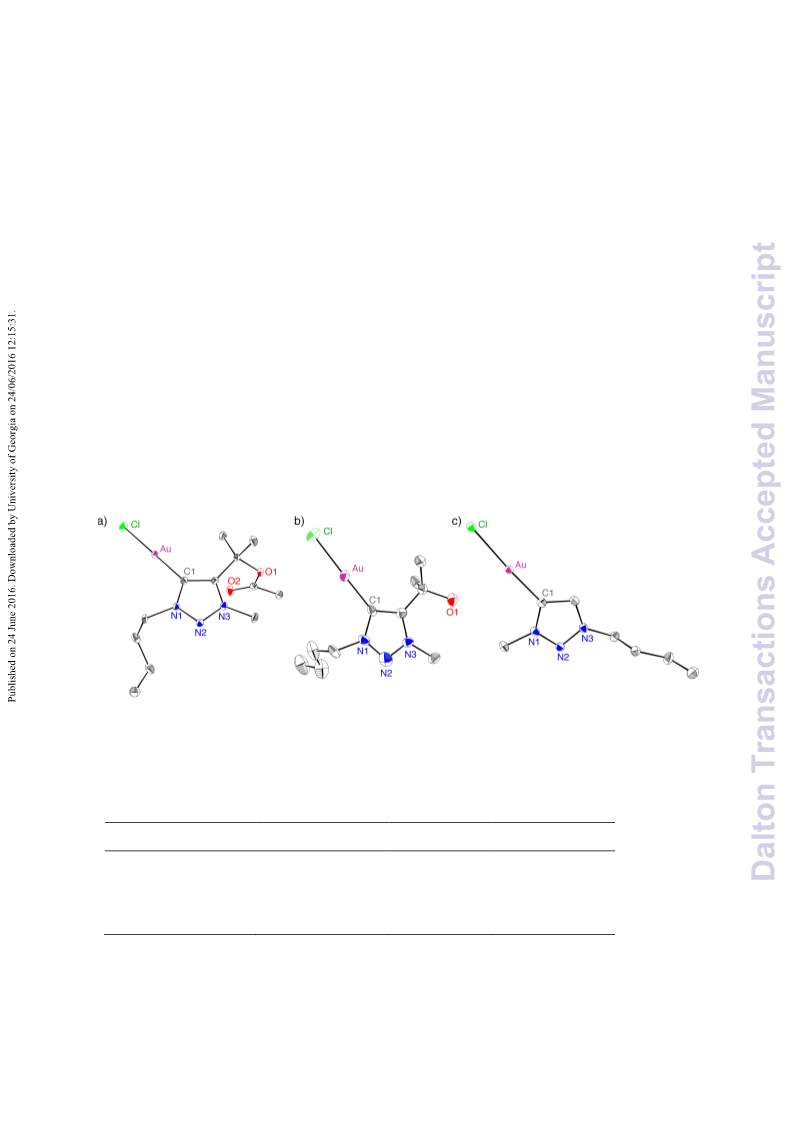
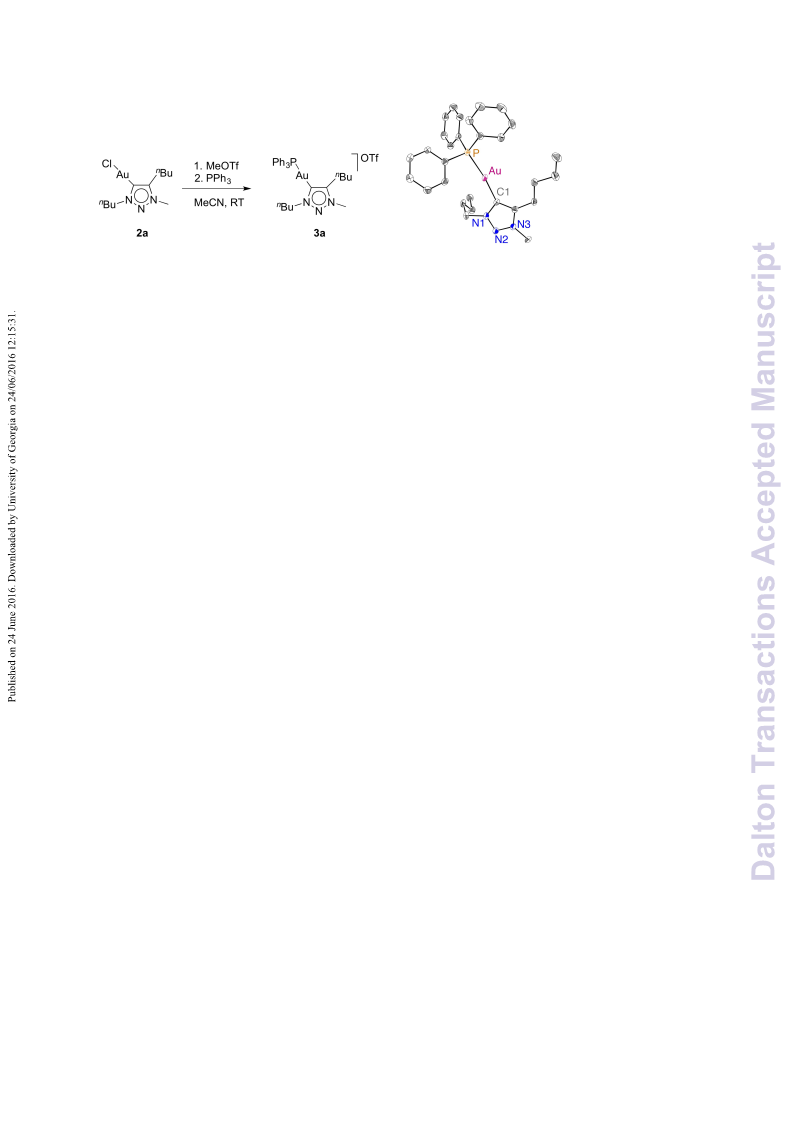
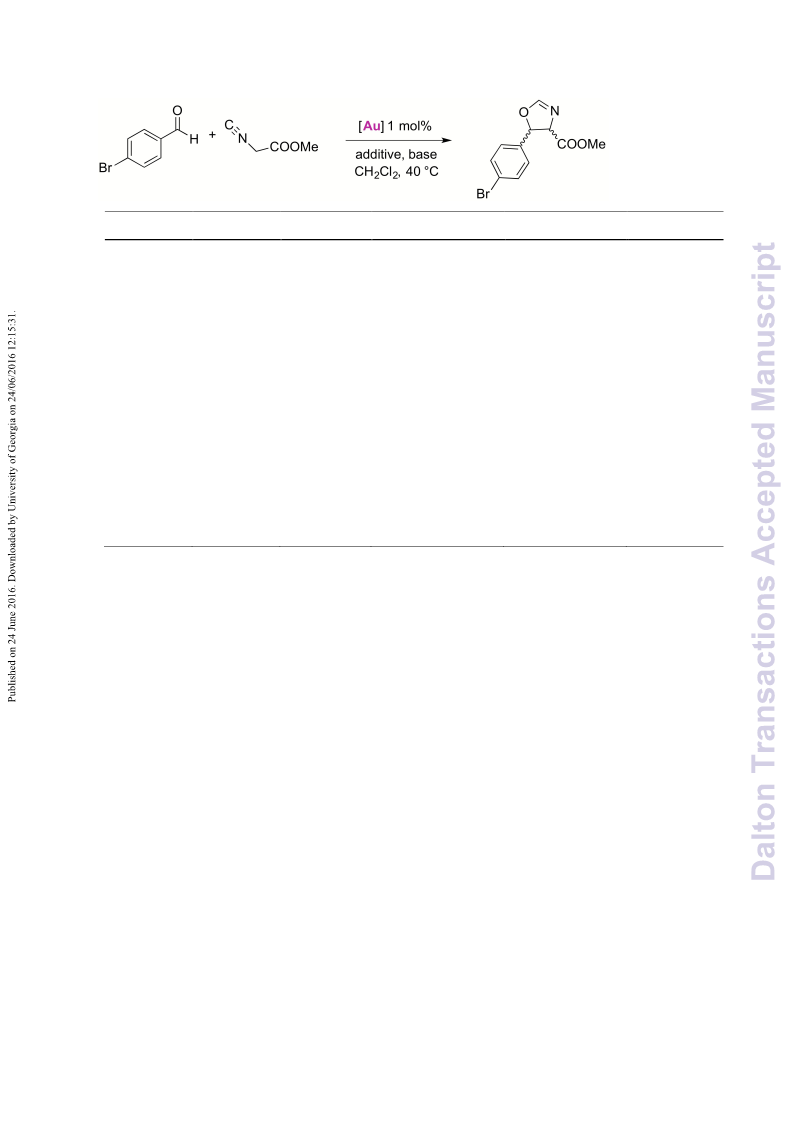
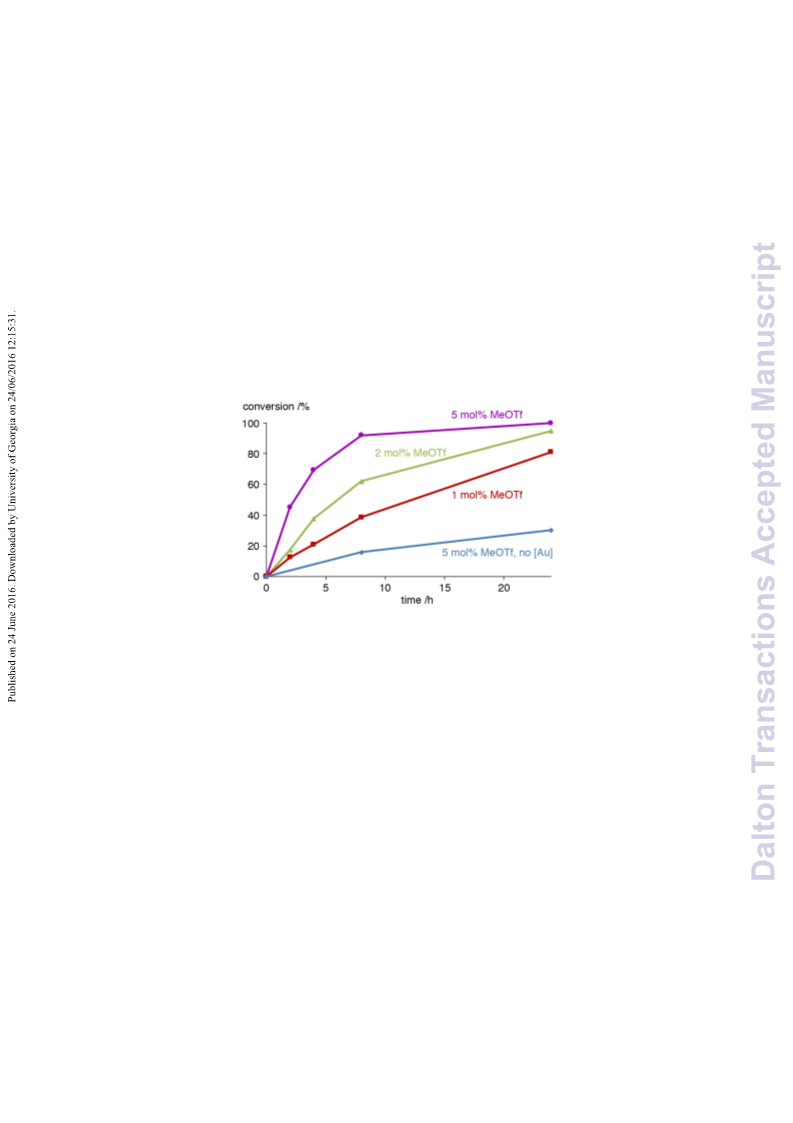
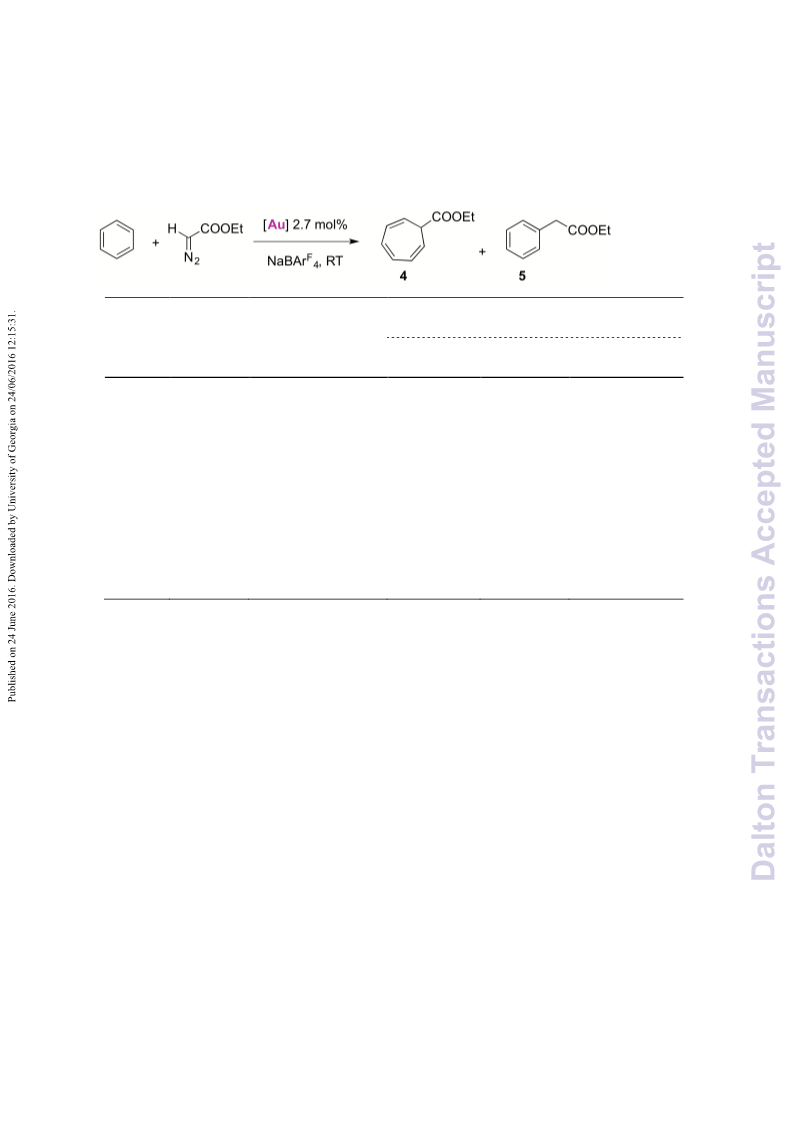
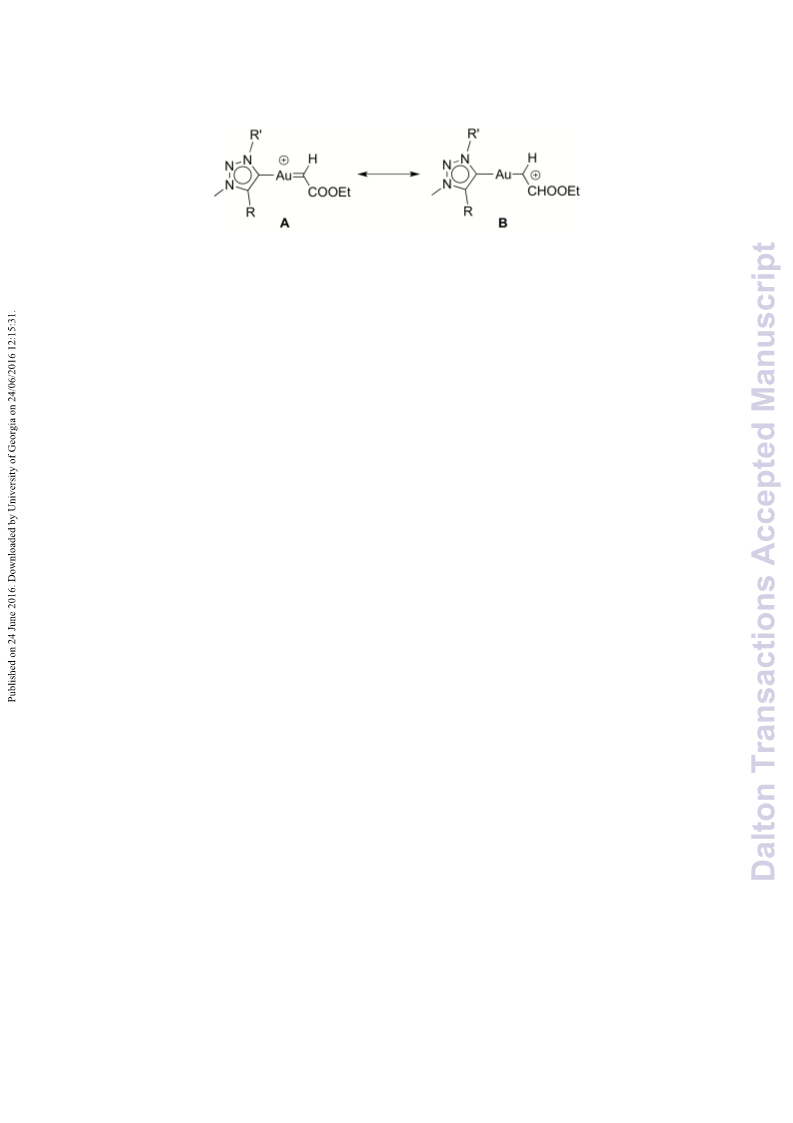
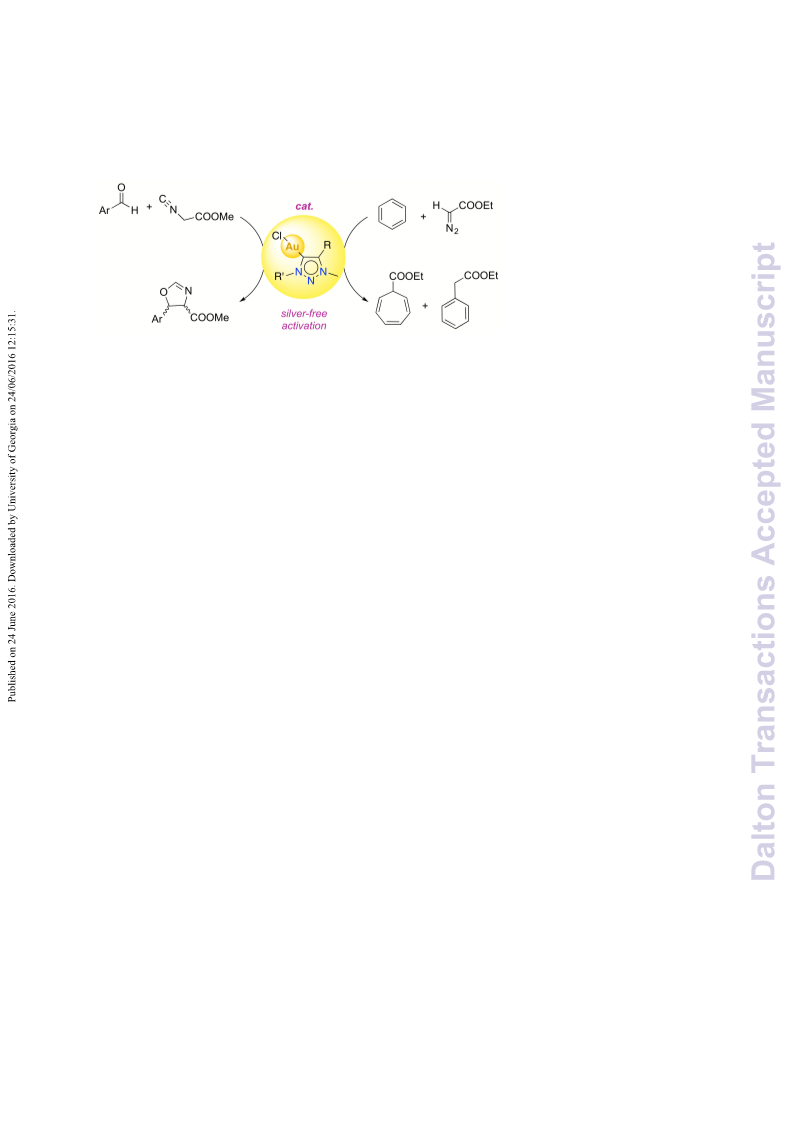
A series of novel 1,2,3-triazolylidene gold(i) chloride complexes have been synthesised and fully characterised. Silver-free methodologies for chloride ion abstraction of these complexes were evaluated for their potential as Au-based catalyst precursors. Using simple potassium salts or MeOTf as chloride scavengers produced metal complexes that catalyse both the regioselective synthesis of oxazolines and the C-H activation of benzene or styrene for carbene transfer from ethyl diazoacetate. These results indicate that Ag-free activation of 1,2,3-triazolylidene gold(i) chloride complexes is feasible for the generation of catalytically active Au triazolylidene species. However, silver-mediated activation imparts substantially higher catalytic activity in oxazoline synthesis.
View More



















Chengdu Green technology Co.,Ltd.
Contact:86-28-82608355
Address:C9 ,Economic Headquarters, Economic Development Zone, Chengdu.
Shanghai Sunwise Chemical Co., Ltd
website:http://www.sunwisechem.com
Contact:86-021-33883180
Address:Room 10E, Building G, Westlink Center, No. 2337 Gudai Road, Minhang District, Shanghai, China PC: 201100
Hebei Think-Do Environment Co., Ltd
website:http://www.thinkdo-environment.com
Contact:0311-86510809
Address:No 6, Shilian Middle Street, Circular Chemical Industry Park
Shandong Hongxiang Zinc Co., Ltd
Contact:086-0311-66187879
Address:DaWang developing zone
Shanghai Sinofluoro Scientific Co., Ltd
Contact:+86-21-64279360
Address:Room 1006,Building 3,#58 East Xinjian Road, Shanghai ,201100,China,
Doi:10.1016/0022-328X(88)80662-4
(1988)Doi:10.1021/acs.inorgchem.5b02921
(2016)Doi:10.1016/j.toxicon.2019.09.018
(2019)Doi:10.1021/jm0612923
(2007)Doi:10.1246/cl.2011.171
(2011)Doi:10.1007/BF01500409
(1933)