
Tetrahedron p. 9033 - 9072 (1992)
Update date:2022-08-11
Topics:
 Foeldesi, Andras
Foeldesi, Andras
 Nilson, Frans Peder R.
Nilson, Frans Peder R.
 Glemarec, Corine
Glemarec, Corine
 Gioeli, Carlo
Gioeli, Carlo
 Chattopadhyaya, Jyoti
Chattopadhyaya, Jyoti
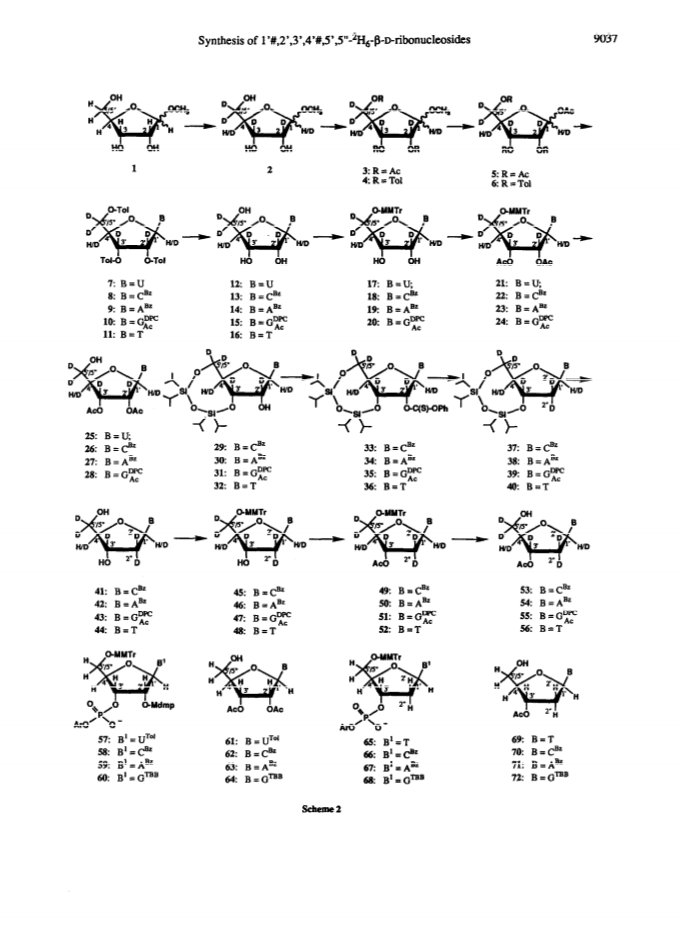
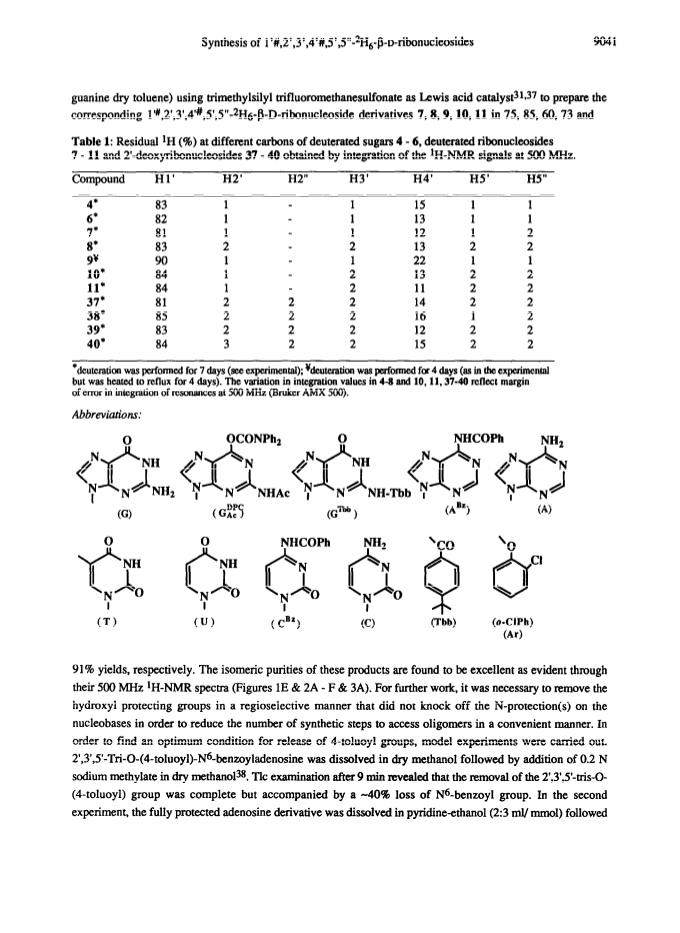
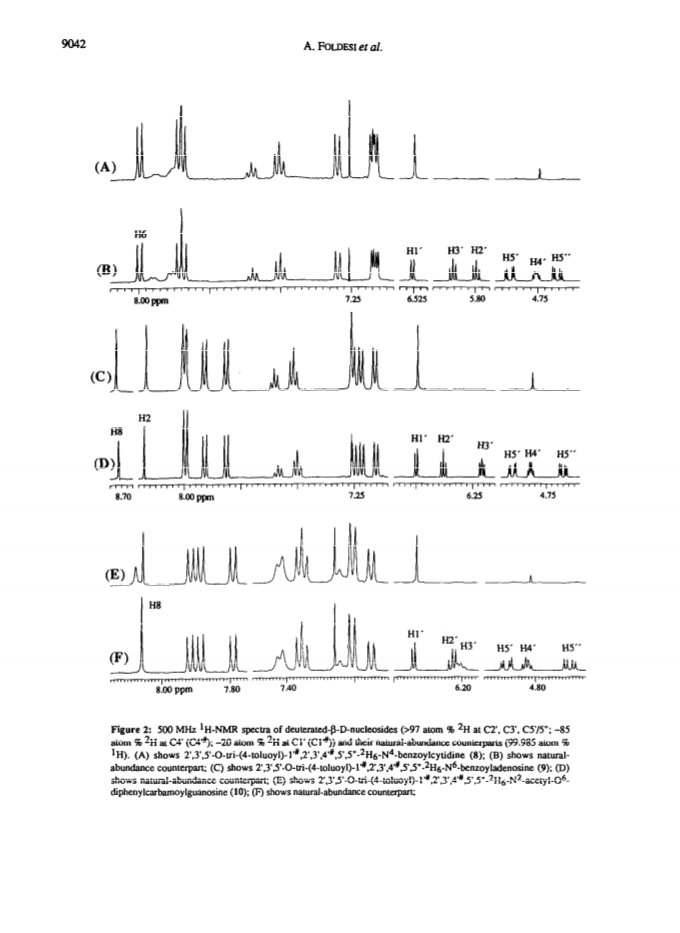
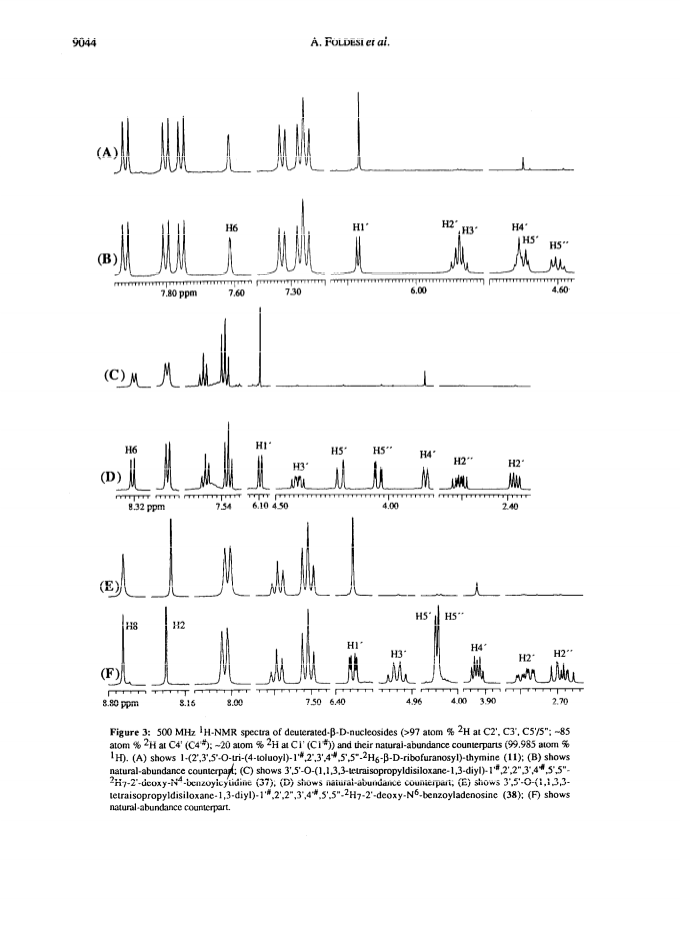
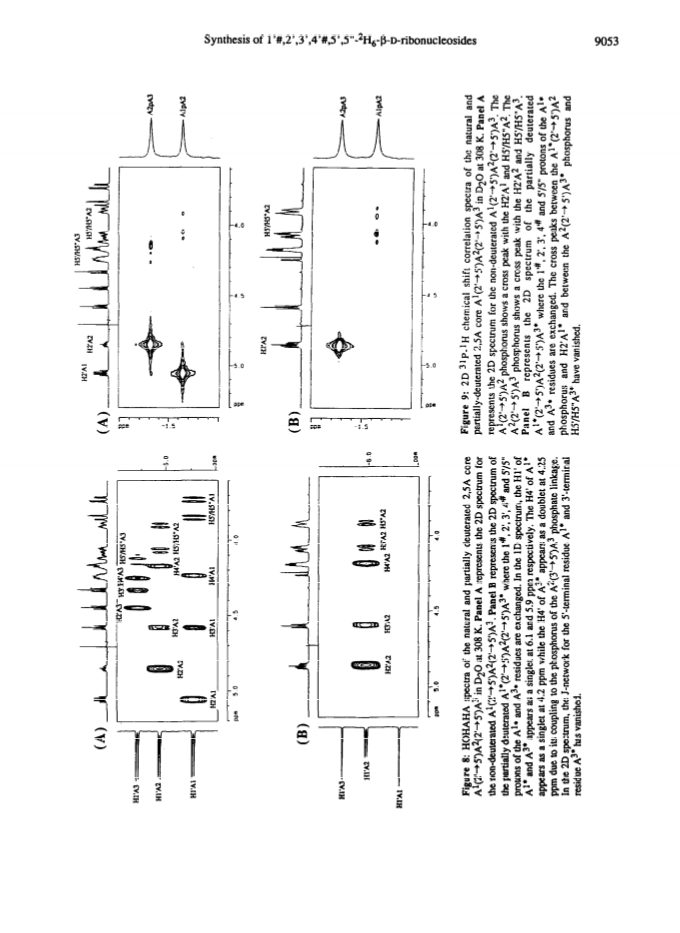
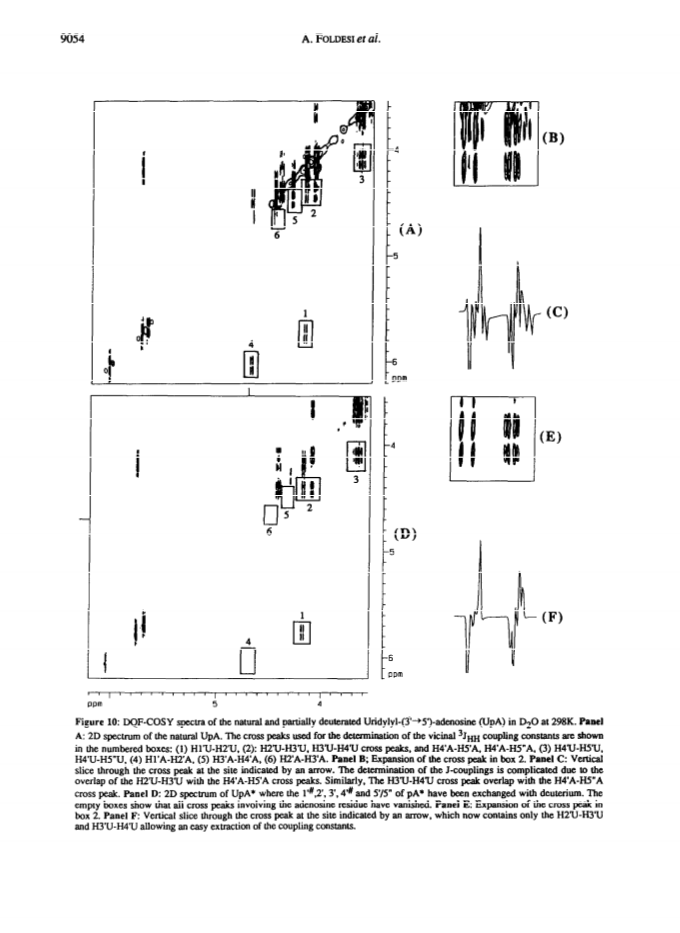
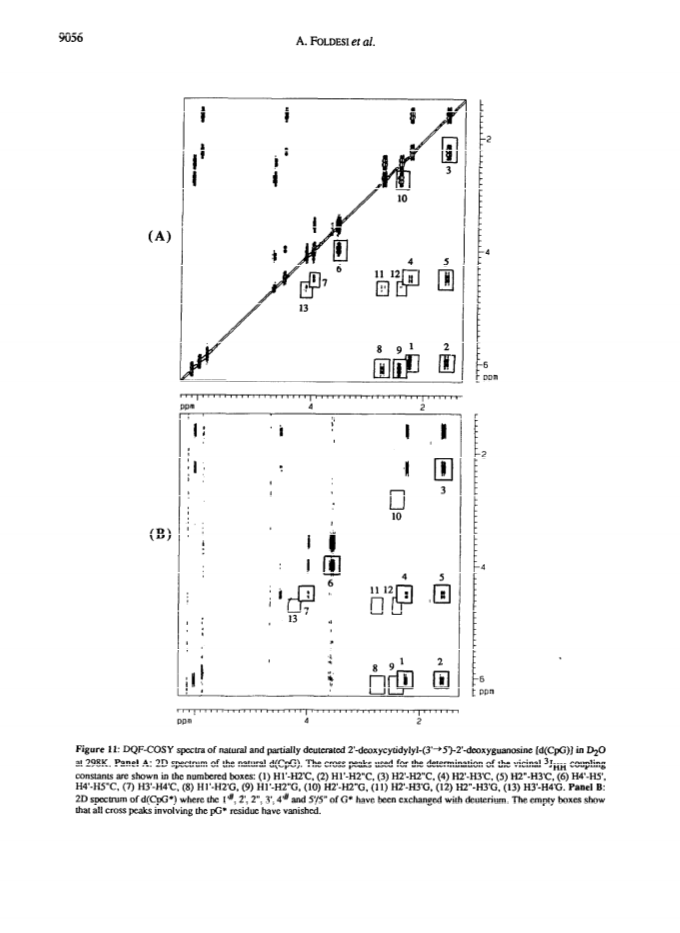
Raney nickel-(2)H2O exchange reaction on an epimeric mixture of methyl α/β-D-ribofuranoside <α/β = ca.3:10> 1 produced methyl 1%,2,3,4%,5,5'-(2)H6-α/β-D-ribofuranoside 2 ( >97 atom percent (2)H at C2, C3, C5/5'; ca. 85 atom percent (2)H at C4(C4%); ca. 20 atom percent (2)H at C1(C1%)) which was obtained in 60 - 80percent yield along with epimeric xylo and arabino by-products.Toluoylation of the crude 2 in dry pyridine and a careful separation on a column of silica gel gave pure 1-O-methyl-2,3,5-tri-O-(4-toluoyl)-α/β-D-1%,2,3,4%,5,5'-(2)H6-ribofuranoside 4 (48percent).Conversion of 4 to1-O-acetyl-2,3,5-tri-O-toluoyl-α/β-D-1%,2,3,4%,5,5'-(2)H6-ribofuranoside 6 (82percent) provided the crucial building block for the synthesis of deuterionucleosides for RNA or DNA synthesis.Compound 6 was then condensed with silyated uracil, N4-benzoylcytosine, N6-benzoyladenine, N2-acetyl-O6-diphenylcarbamoylguanine and thymine in anhydrous solvent using trimethylsilyl trifluoromethanesulfonate to give the corresponding isomerically pure 1'%,2',3',4'%,5',5"-(2)H6-ribonucleoside derivatives 7, 8, 9, 10, 11 in 75, 85, 60, 73 and 91percent yields, respectively. 1'%,2',3',4'%,5',5"-(2)H6-ribonucleosides 13-16 were converted in high yields to the corresponding 1'%,2',2",3',4'%,5',5"-(2)H7-2'-deoxynucleosides 41-44 in the following manner: 3',5'-O-(1,1,3,3-tetraisopropyldisiloxane-1,3-diyl (TPDS)-1'%,2',3',4'%,5',5"-(2)H6-nucleosides 29-32 were converted to the corresponding 2'-O-phenoxythiocarbonyl derivatives 33-36, which were deoxygenated by tri-n-butyltin deuteride to give 1'%,2',2",3',4'%,5',5"-(2)H7-2'-deoxynucleosides 37-40 and subsequently deprotected to give 41-44.Pure 1'%,2',3',4'%,5',5"-(2)H6-ribonucleoside derivatives 12-15, 1'%,2',2",3',4'%,5',5"-(2)H7-2'-deoxynucleoside blocks 41-44 and their natural-abundance counterparts were then used to assemble partially deuterated ribonucleotide-dimers (* indicates deuterated moiety): UpA* 77, CpG* 78, ApU* 79, GpC* 80, partially deuterated 2'-deoxyribonucleotide-dimers d(TpA*) 93, d(CpG*) 94, d(ApT*) 95, d(GpC*) 96 and partially deuterated 2,5A core (A*2'p5'A2'p5'A*) (109).These nine partially deuterated oligonucleotides were subsequently compared with their corresponding natural-abundance counterparts by 500 MHz (1)H-NMR spectroscopy to evaluate the actual NMR simplifications achieved in the non-deuterated part ((1)H-NMR window) as a result of specific deuterium incorporation.Detailed 1D (1)H-NMR (500 MHz), 2D correlation spectra (DQF-COSY and TOCSY), T1 measurements for (1)H-, (13)C- and INEPT (13)C-NMR spectra have been presented and discussed to assess the utility of stereospecific deuterium incorporation to create the (1)H- or (13)C-NMR window.
View More





















TIANJIN FESTO CHEMICAL CO.,LTD(expird)
Contact:86-22-25814570
Address:No.12th,5th Ave.,TEDA,Tianjin,China
Contact:86-574-26865651
Address:529 YuanBaoShan Road, Beilun District
Zhejiang kehong chemical co., ltd
Contact:0086-575-85522000
Address:xiner center RD binhai industrial zone shaoxing zhejiang province P.R.China,312073
website:http://www.lonwinchem.com
Contact:Tel: 86-21-59858395
Address:No#966,Huaxu Road,Shanghai 201702,P.R.China
website:http://www.angchenchem.com
Contact:+86-510-88302099 82327577
Address:Rm. 404/405, Floor 4th, No. 983 FengXiang Road, Wuxi, China
Doi:10.1016/0957-4166(95)00322-G
(1995)Doi:10.1039/c5ob01343g
(2015)Doi:10.1021/om500485m
(2014)Doi:10.1016/j.materresbull.2011.02.037
(2011)Doi:10.1002/jlcr.3511
(2017)Doi:10.1021/om500922n
(2014)