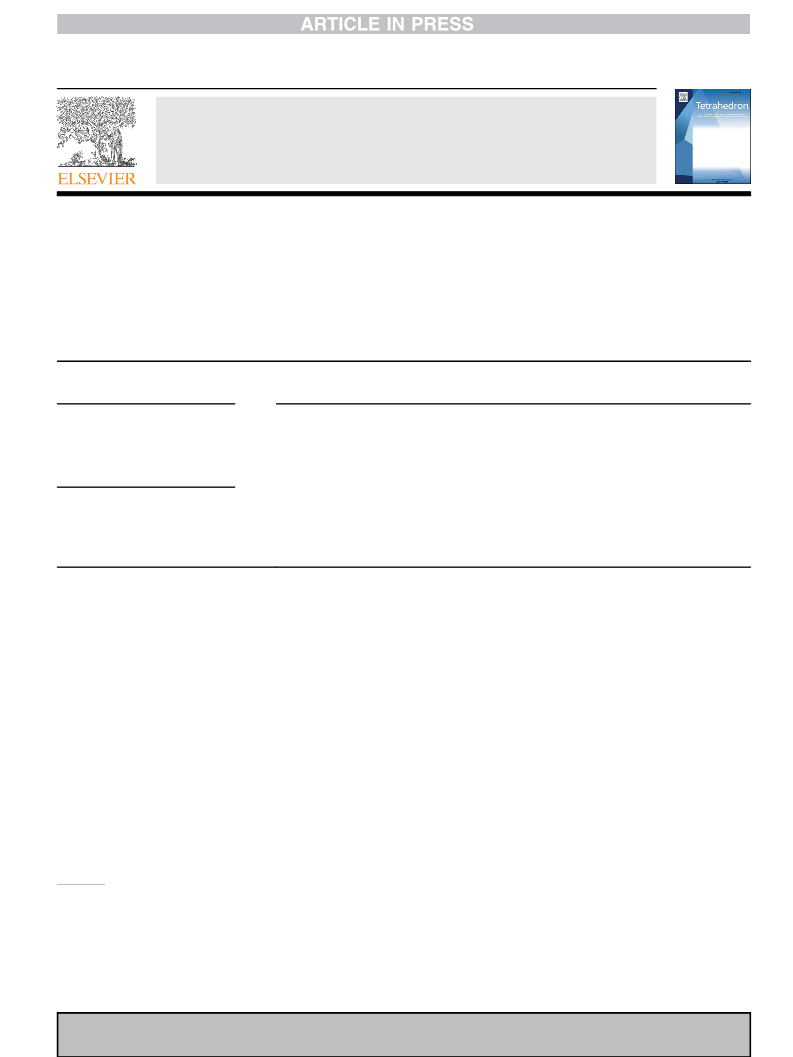
4
B. Ticconi et al. / Tetrahedron xxx (xxxx) xxx
determines the reactivity of the different amino acidic substrates
[
7,8]. The lower selectivity observed with PINO compared to that
ꢁ
observed with Br , could be explained with a lower degree of CeH
bond cleavage in the TS with the former radical with respect to the
latter [7,8].
An opposite regioselectivity is instead observed in HAT pro-
ꢁ
moted by HO as compared to that found with PINO. Highly reactive
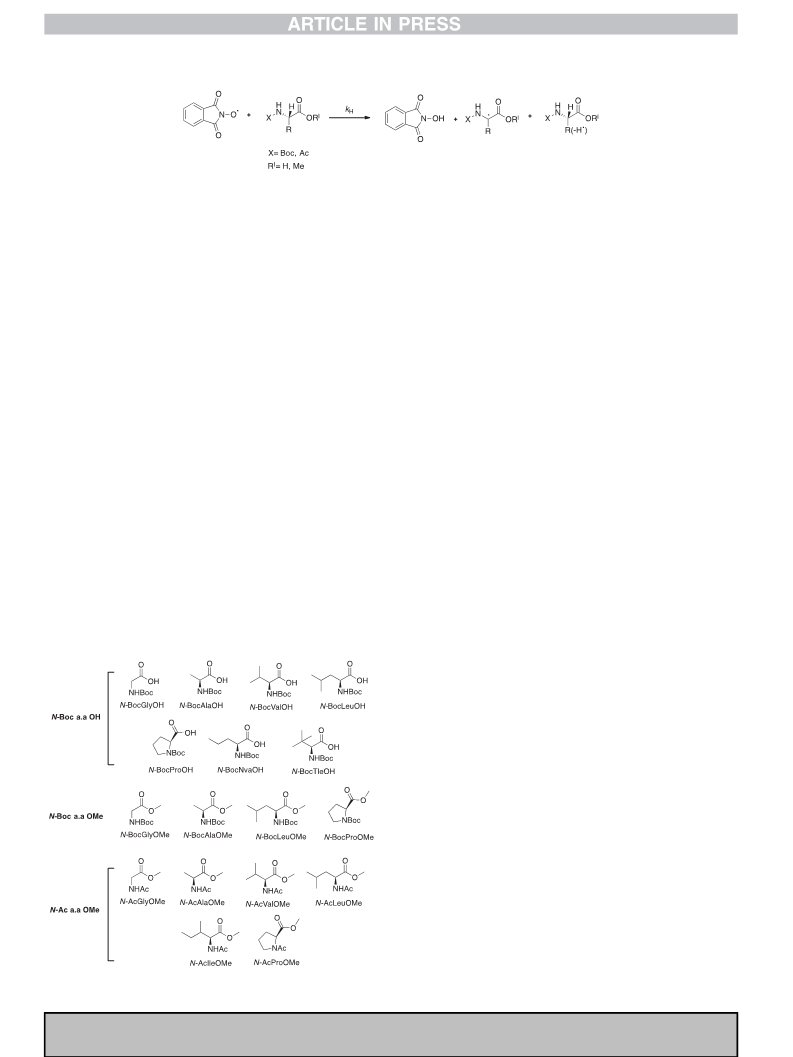
Fig. 3. Steric hindrance in the Ca radicals of N-BocAlaOH (a), N-BocTleOH (b) and N-
BocValOH (c).
ꢁ
OH was found to promote HAT preferentially from CeH bonds of
the side chain. This regioselectivity was rationalized on the basis of
an “early” TS. In this scenario, a hypothetical HAT from the Ca-H
and
be explained on the basis of polar effects, since both NHBoc and
CO H groups exert a deactivating effect on HAT processes from CeH
bonds of the side chain to PINO radical. The lower k values
determined with N-BocValOH and N-BocTleOH can be explained by
the presence of steric effects. With these substrates a drop of
reactivity of about 4e6 times is observed when compared to N-
BocAlaOH. The steric interaction of the bulky isopropyl and the tert-
butyl side chains of N-BocValOH and N-BocTleOH with the car-
boxylic and carbamate groups hinders the planarization of the
carbon centered radical generated in the HAT process and therefore
the captodative stabilization of these radicals (Fig. 3b and c). Under
these conditions it is not possible to have an optimal overlap be-
d
positions of the side chain. This marked regioselectivity can
bond is less influenced by the thermodynamic stability of the
incoming radical but is affected by the presence of the adjacent
2
electron-withdrawing groups which deactivate the C
wards a HAT process promoted by the electrophilic OH radical
[2,4,27].
a
-H bond to-
ꢁ
H
Among all the N-Boc-protected amino acids investigated, N-
BocProOH showed the highest k value for the HAT process to PINO
H
ꢀ
1
ꢀ1
(0.56 M
s
), about 6 times higher than that obtained with N-
BocGlyOH. Differently from the other amino acids, N-BocProOH has
two different reactive sites adjacent to the nitrogen atom indicating
that the HAT process can occur not only at C -H bond, but also at C -
a
d
H bonds leading to the formation of two radicals as shown in Fig. 4.
Both the C ꢀH and the C ꢀH bonds benefit from the activation
a
d
tween the p-orbital on C
a
and the
p
orbitals of the carbamate and
provided by the adjacent carbamate group, however, at the same
carbonyl groups in the radical product [8,26].
In order to obtain information about the role of the hydrogen-
abstracting radical nature in the HAT process from amino acids, it
time, C ꢀH bond is deactivated by the electron withdrawing
a
character of the carboxylic group. The remarkable increase in
reactivity observed with N-BocProOH suggests that, unlike the
other acyclic amino acids seen so far, the HAT process may involve
the C ꢀH bond rather than the C ꢀH bond.
is interesting to compare the k
those determined for HAT process promoted by other radical spe-
cies. Comparing the relative reactivity k (X)/k (Gly) displayed in
H
values measured in this work with
d
a
H
H
In addition to the polar effects discussed above, steric effects
Table 3, it is possible to note that the results obtained in our kinetic
studies follow the same behavior of those recently obtained for the
may also be responsible for the observed HAT regioselectivity in N-
BocProOH. Steric effects prevent the planarization of the
a carbon
study of HAT process from N-Boc protected
cumyloxyl radical (CumO ) with the exception of N-BocLeuOH for
a
-amino acids to
centered radical (Fig. 4B) and does not allow its stabilization by
delocalization of the unpaired electron on the adjacent carbamate
ꢁ
which a significantly higher relative reactivity was observed with
and carbonyl groups, which is instead possible for the d carbon
ꢁ
CumO likely indicating an involvement of the C
side chain in the HAT process [9]. This behavior is different from
that found in HAT process promoted by PINO where a regioselective
g
ꢀH bond of the
centered radical (Fig. 4A) [8]. In support to this hypothesis, previous
studies concerning HAT processes from CeH bonds of amides have
confirmed that the result obtained with N-BocProOH can be
reasonably explained on the basis of stereoelectronic effects.
Accordingly, HAT reactions from CeH bonds of amides to PINO
HAT from C
a
-H bond is observed. Such a different behavior might
ꢁ
be due to the much greater stability of PINO with respect to CumO .
ꢁ
Concerning HAT process from amino acids to Br , data in Table 3
were found to be faster when the Ca-H bond is collinear with the p
show that it is strongly influenced by steric effects: increasing the
steric hindrance of the amino acid side chain a decrease of relative
rates was observed. With this radical, HAT process exclusively in-
amide system. This orbital overlap weakens the adjacent CeH bond
and stabilizes the carbon radical generated in HAT process [21].
With N-BocProOH the steric restrictions imposed by the 5-
volves C
a
-H bond and a “late” transition state (TS), in which CeH
membered heterocyclic ring force the CeH bonds in
the nitrogen to assume a collinear position to the system of the
carbamate group, making them more activated by stereoelectronic
effect towards HAT process with respect to the C -H bond of the
other acyclic -amino acids described above (Fig. 5).
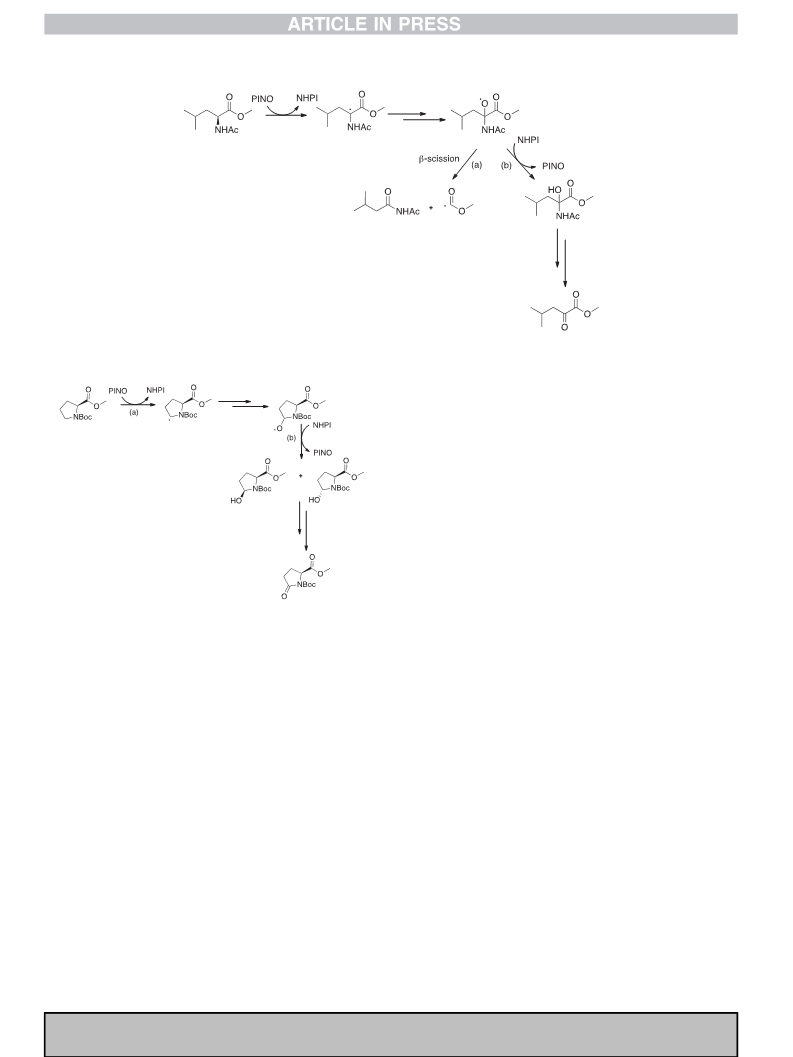
As previously mentioned, a significant advantage of HAT pro-
cesses promoted by PINO resides in the possibility to demonstrate
the regioselectivity hypotheses based on kinetic data, by investi-
gating the product distribution in the corresponding aerobic
oxidation under Ishii conditions. Thus, to confirm that HAT process
d position to
ꢁ
bond cleavage is advanced, has been proposed. With both Br and
PINO the relative stability of the
p
a-carbon centered radical
a
a
Table 3
H H
Relative Rates (k (X)/k (Gly)) for HAT processes from amino acids to different
radical species.
PINOa
CumOꢁ
b
Brꢁ
c
OHꢁ
d
Amino acid
Gly
Ala
Val
Nva
Tle
1.0
0.8
0.2
0.6
0.1
0.8
5.7
1.0
0.7
0.5
0.8
0.6
1.5
6.3
1.0
0.33
0.04
1.0
1.1
3.7
9.7
4.3
9.1
promoted by PINO involves the C
aꢀH bond with acyclic amino
<4 ꢃ 10ꢀ
4
Leu
Pro
1.4
a
This work.
b
N- tert-butoxycarbonyl protected amino acids, 266 nm laser flash photolysis,
ꢂ
T ¼ 25 C, MeCN, dicumyl peroxide 10 mM [6].
c
N-Benzoylated amino acids, steady-state photolysis, T ¼ 25 ꢂC in CCl
4
containing
N-bromosuccinimide [5a,b].
d
N-Acetylated amino acids, steady-state photolysis, T ¼ 25 ꢂC in D
2
O acidified
with TFA [4,8].
Fig. 4. Possible radicals formed in the HAT process from N-BocProOH.
Please cite this article as: B. Ticconi et al., Oxidation of
Tetrahedron, https://doi.org/10.1016/j.tet.2019.05.026
a-amino acids promoted by the phthalimide N-oxyl radical: A kinetic and product study,

 Ticconi, Barbara
Ticconi, Barbara
 Mazzonna, Marco
Mazzonna, Marco
 Lanzalunga, Osvaldo
Lanzalunga, Osvaldo
 Lapi, Andrea
Lapi, Andrea