
Biochemistry p. 611 - 622 (2010)
Update date:2022-08-16
Topics:
 Cummings, Jennifer A.
Cummings, Jennifer A.
 Nguyen, Tinh T.
Nguyen, Tinh T.
 Fedorov, Alexander A.
Fedorov, Alexander A.
 Kolb, Peter
Kolb, Peter
 Xu, Chengfu
Xu, Chengfu
 Fedorov, Elena V.
Fedorov, Elena V.
 Shoichet, Brian K.
Shoichet, Brian K.
 Barondeau, David P.
Barondeau, David P.
 Almo, Steven C.
Almo, Steven C.
 Raushel, Frank M.
Raushel, Frank M.
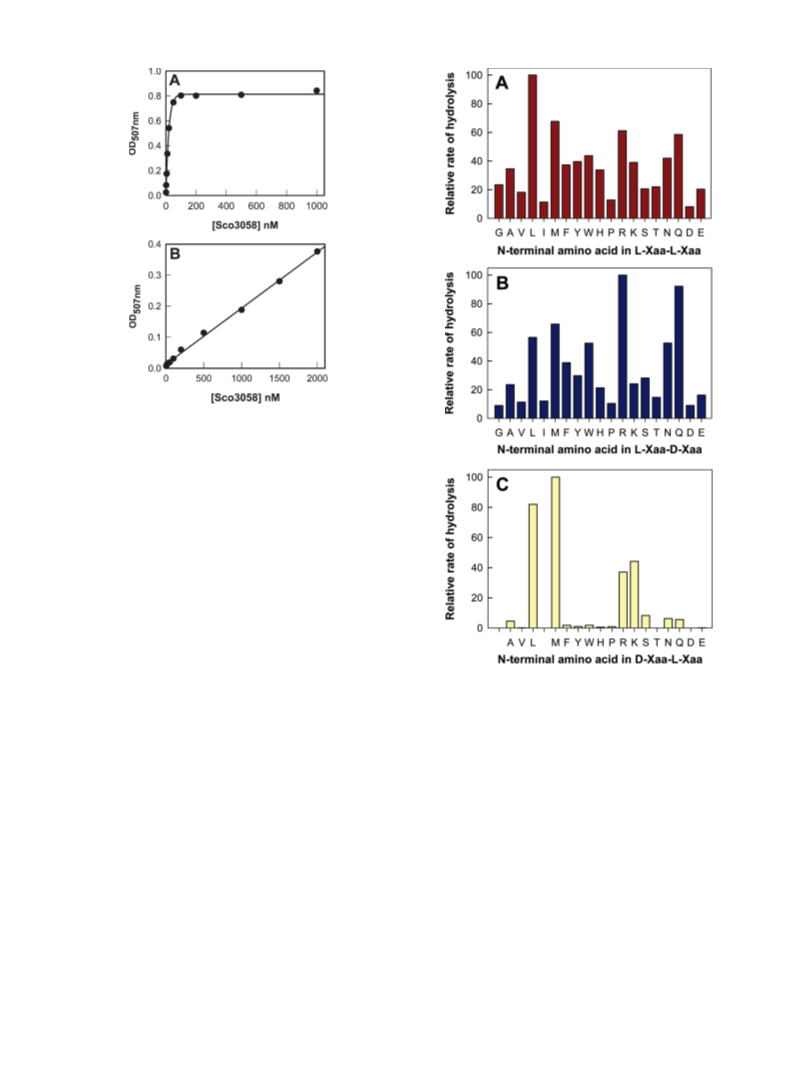
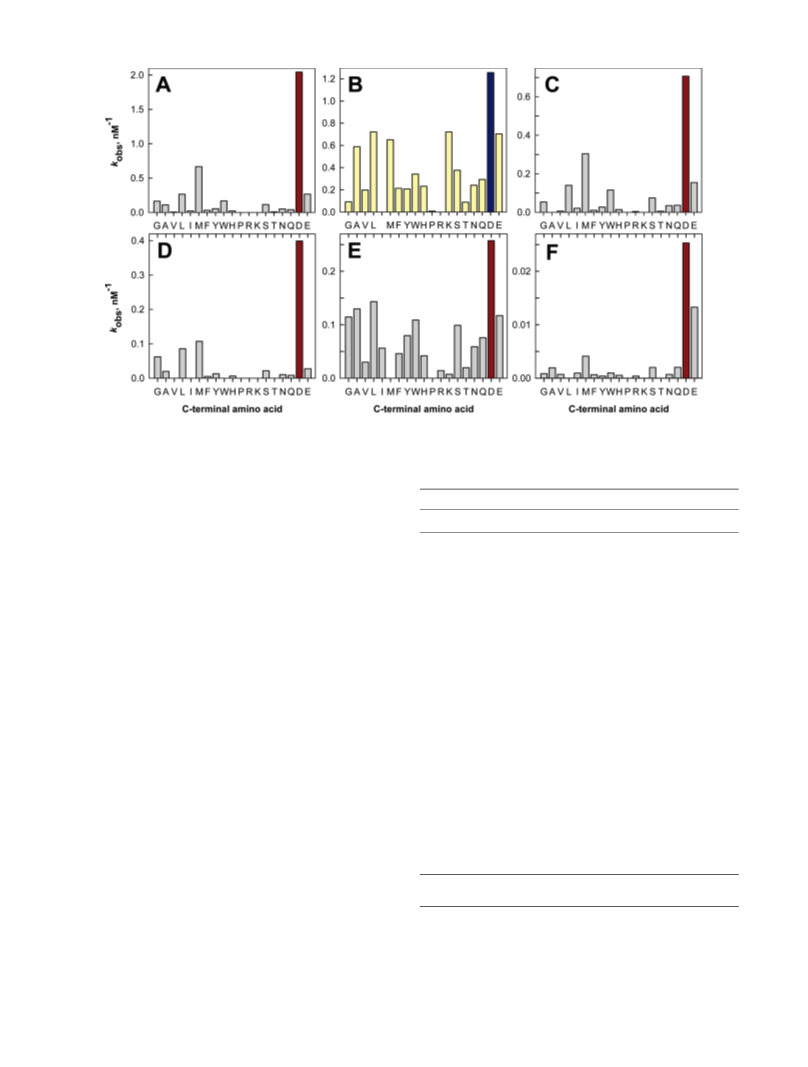
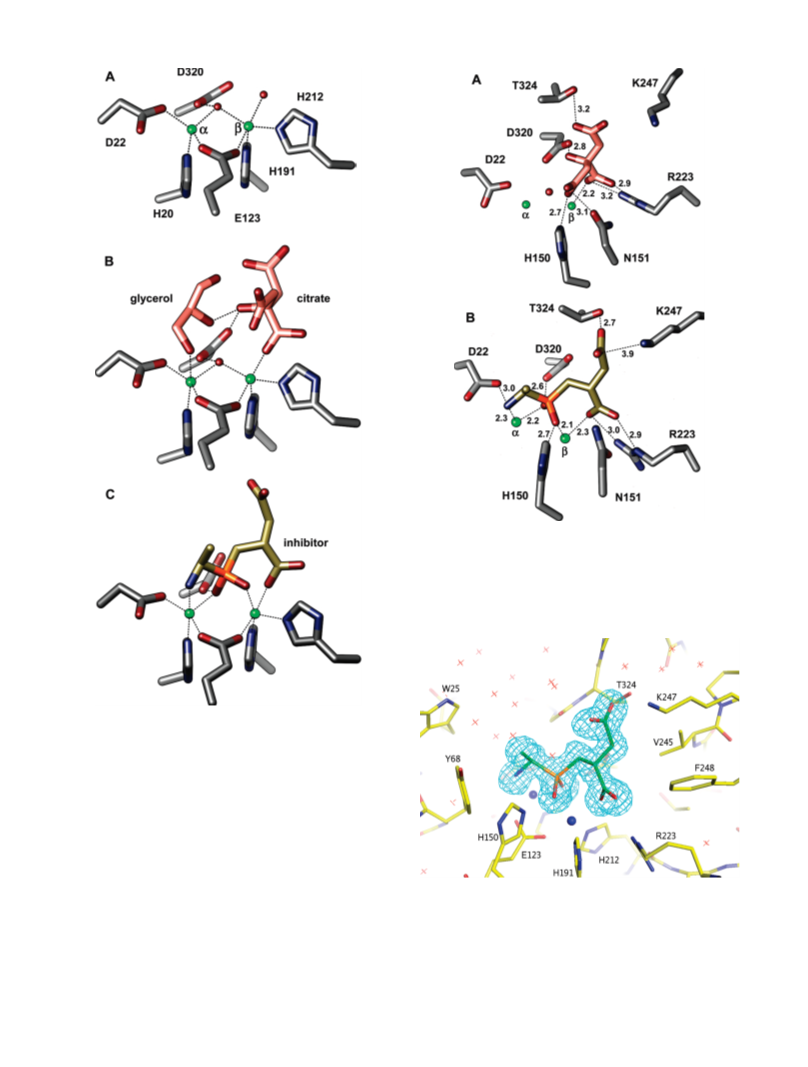
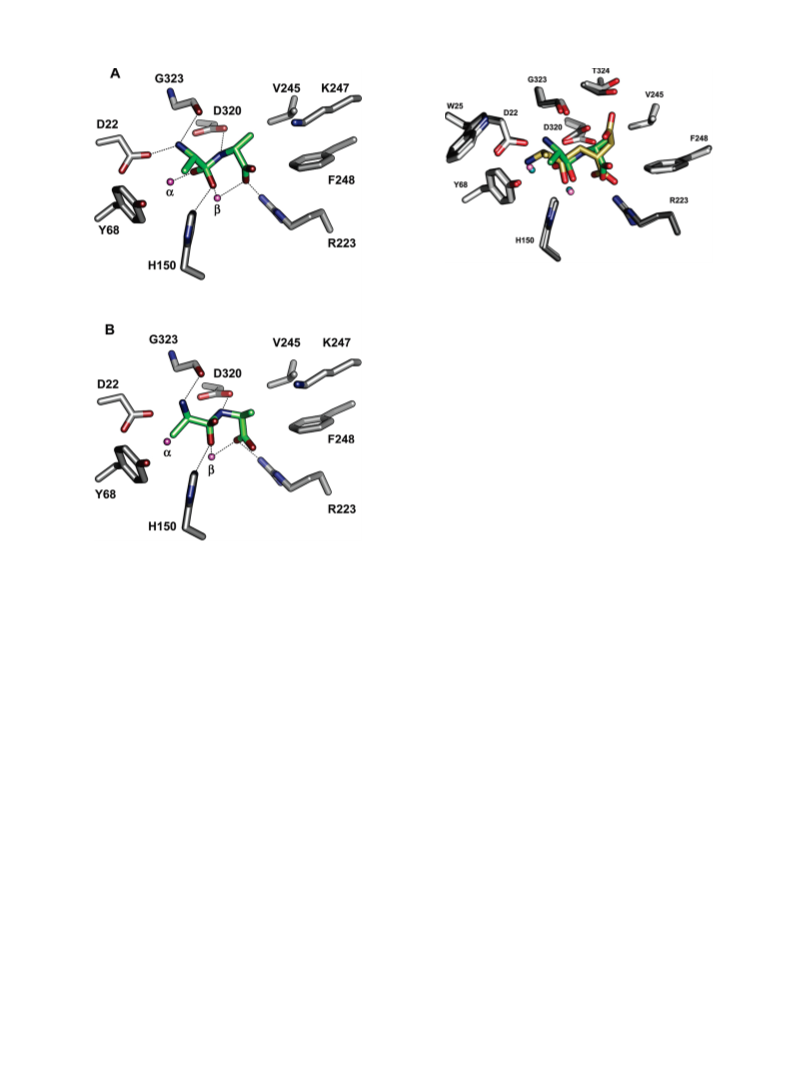
Human renal dipeptidase, an enzyme associated with glutathione metabolism and the hydrolysis of β-lactams, is similar in sequence to a cluster of ~400 microbial proteins currently annotated as nonspecific dipeptidases within the amidohydrolase superfamily. The closest homologue to the human renal dipeptidase from a fully sequenced microbe is Sco3058 from Streptomyces coelicolor. Dipeptide substrates of Sco3058 were identified by screening a comprehensive series of L-Xaa-L-Xaa, L-Xaa-D-Xaa, and D-Xaa-L-Xaa dipeptide libraries. The substrate specificity profile shows that Sco3058 hydrolyzes a broad range of dipeptides with a marked preference for an L-amino acid at the N-terminus and a D-amino acid at the C-terminus. The best substrate identified was L-Arg-D-Asp (kcat/Km = 7.6 x 105 M -1 s-1). The three-dimensional structure of Sco3058 was determined in the absence and presence of the inhibitors citrate and a phosphinate mimic of L-Ala-D-Asp. The enzyme folds as a (β/α)8 barrel, and two zinc ions are bound in the active site. Site-directed mutagenesis was used to probe the importance of specific residues that have direct interactions with the substrate analogues in the active site (Asp-22, His-150, Arg-223, and Asp-320). The solvent viscosity and kinetic effects of D2O indicate that substrate binding is relatively sticky and that proton transfers do not occurr during the rate-limiting step. A bell-shaped pH-rate profile for kcat and kcat/Km indicated that one group needs to be deprotonated and a second group must be protonated for optimal turnover. Computational docking of high-energy intermediate forms of L/D-Ala-L/D-Ala to the three-dimensional structure of Sco3058 identified the structural determinants for the stereochemical preferences for substrate binding and turnover.
View More







Shanghai Kangxin Chemical Co., Ltd
Contact:+86 21 60717227
Address:118,Ganbai Village,Waigang Town,Jiading District,Shanghai
ANHUI CHEM-BRIGHT BIOENGINEERING CO.,LTD
Contact:86-561-4080321
Address:No.8 Lieshan Industrial Zone of Huaibei
SHANXI JINJIN CHEMICAL INDUSTRIAL CO.,LTD
website:http://www.jinjingroup.com
Contact:4009982989
Address:Economic And Technological Development Zone,Hejin?City,Shanxi Province?,China
website:http://www.hope-chem.com
Contact:86-21-58090396-805
Address:Floor 4, Building 5, No.588 Tianxiong Road, Zhoupu International Medical Zone, ShangHai, China
Hangzhou Mole's Science & Technology Co.,Ltd.(expird)
Contact:+86-571-56880228
Address:15F Guodu development Building, NO.182 Zhaohui Road
Doi:10.3390/molecules16021825
(2011)Doi:10.1039/c9cc09466k
(2020)Doi:10.1021/j150576a048
(1959)Doi:10.1007/BF00564314
()Doi:10.1021/ic202342v
(2012)Doi:10.1021/ie50518a022
(1953)