- Neomycin
- Neomycin A
- Neomycin B
- Neomycin B sulphate
- Neomycin c
- NEOMYCIN E
- Neomycin sulfate
- 39478-78-9Benzenamine,5-bromo-2-methyl-
- 39482-87-6Silanamine,N-[dimethyl(3,3,3-trifluoropropyl)silyl]-1,1-dimethyl-1-(3,3,3-trifluoropropyl)-
- 39483-74-4Bismuthine, trichloro-,monohydrate (9CI)
- 39489-77-5Phenol,2,4-dichloro-5-nitro-
- 39489-79-7Phenol,5-amino-2,4-dichloro-
- 39489-86-62-Butanone,1-(4-chlorophenyl)-3,3-dimethyl-
- 39492-01-8Benzoic acid,4-[[6-[(aminoiminomethyl)amino]-1-oxohexyl]oxy]-, ethyl ester
- 3949-36-82H-1-Benzopyran-2-one,3-acetyl-
- 3949-47-1Phosphorothioictriamide, hexabutyl- (7CI,8CI,9CI)
- 394-96-75-Chloro-3-fluorosalicylaldehyde
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Neomycin A |
EINECS | N/A |
| CAS No. | 3947-65-7 | Density | 1.51g/cm3 |
| PSA | 203.46000 | LogP | -2.31440 |
| Solubility | N/A | Melting Point |
225.5°C (rough estimate) |
| Formula | C12H26 N4 O6 | Boiling Point | 577.9°C at 760 mmHg |
| Molecular Weight | 322.362 | Flash Point | 303.3°C |
| Transport Information | N/A | Appearance | N/A |
| Safety | Poison by intravenous route. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx. | Risk Codes | N/A |
| Molecular Structure |
|
Hazard Symbols | N/A |
| Synonyms |
NeomycinA (6CI,7CI,8CI); (+)-Neamine; Dekamycin V; Neamin; Neamine; Nebramycin X; Negamicin;O-2,6-Diamino-2,6-dideoxy-a-D-glucopyranosyl-(1®4)-1,3-diamino-1,2,3-trideoxy-D-myo-inositol; ST 7 |
Article Data | 20 |
Neomycin A Synthetic route
- 549501-35-1
(1S,2R,3R,4S,6R)-4,6-Diamino-3-((2R,3R,4R,5R,6R)-3-amino-6-aminomethyl-4,5-bis-benzyloxy-tetrahydro-pyran-2-yloxy)-cyclohexane-1,2-diol

- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| With hydrogen; 20% palladium hydroxide on carbon In water; acetic acid | 91% |
| With hydrogen; acetic acid; palladium dihydroxide |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 6h; Heating; | 82% |
| With hydrogenchloride In methanol for 4h; Reflux; | 80% |
| Stage #1: neomycin B With hydrogenchloride; methanol for 16h; acidic methanolysis; Stage #2: With Amberlite IRA-400 (OH-) In methanol for 0.5h; Hydrolysis; | 67% |
| With water Acidic conditions; Inert atmosphere; |

- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| Stage #1: neomycin B trisulfate With hydrogenchloride In methanol for 6h; Heating; Stage #2: With ammonium hydroxide; ammonia In methanol Further stages.; | 70% |
- 4146-30-9, 25389-98-4, 28002-70-2, 34289-38-8, 92283-95-9
neomycin B trisulfate

- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 80℃; for 3h; pH=< 1; | 67% |
- 25389-98-4
neomycin B sulphate

A
- 98-01-1
furfural

B
- 36468-53-8
β-D-ribofuranose

C
- 3947-65-7
neomycin A

D
- 59433-00-0
2,6-dideoxy-2,6-diamino-D-glucose

| Conditions | Yield |
|---|---|
| Product distribution; Rate constant; Irradiation; multistep reaction, radiolytic degradation; |


| Conditions | Yield |
|---|---|
| Product distribution; Rate constant; Irradiation; multistep reaction, radiolytic degradation; |


- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methanol | |
| With hydrogenchloride |
- 171032-74-9
1,3,4,6-tetra-O-acetyl-2-azido-2-deoxy-D-glucopyranose

- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 90 percent / ZnI2 / 1,2-dichloro-ethane / 50 °C 2: 98 percent / NaOMe / methanol 3: 94 percent / pyridine 4: 100 percent / NaN3 / dimethylformamide / 80 °C 5: 86 percent / NaH / dimethylformamide 6: N-iodosuccinimide; silver trifluoromethanesulfonate / diethyl ether; CH2Cl2 / -20 °C 7: NaOMe / methanol 8: P(CH3)3; 0.1 N aq. NaOH / tetrahydrofuran 9: H2; aq. AcOH / 20 percent Pd(OH)2/C View Scheme |
- 202462-37-1
phenyl 2-azido-2-deoxy-1-thio-α/β-D-glucopyranoside

- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 94 percent / pyridine 2: 100 percent / NaN3 / dimethylformamide / 80 °C 3: 86 percent / NaH / dimethylformamide 4: N-iodosuccinimide; silver trifluoromethanesulfonate / diethyl ether; CH2Cl2 / -20 °C 5: NaOMe / methanol 6: P(CH3)3; 0.1 N aq. NaOH / tetrahydrofuran 7: H2; aq. AcOH / 20 percent Pd(OH)2/C View Scheme |
- 183875-22-1
phenyl 2-azido-2-deoxy-3,4,6-tri-O-acetyl-1-thio-α/β-D-glucopyranoside

- 3947-65-7
neomycin A

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 98 percent / NaOMe / methanol 2: 94 percent / pyridine 3: 100 percent / NaN3 / dimethylformamide / 80 °C 4: 86 percent / NaH / dimethylformamide 5: N-iodosuccinimide; silver trifluoromethanesulfonate / diethyl ether; CH2Cl2 / -20 °C 6: NaOMe / methanol 7: P(CH3)3; 0.1 N aq. NaOH / tetrahydrofuran 8: H2; aq. AcOH / 20 percent Pd(OH)2/C View Scheme |
Neomycin A Chemical Properties
Product Name: Neomycin A
CAS: 3947-65-7
The Molecular formula of Neomycin A (CAS NO.3947-65-7): C12H26N4O6
The Molecular Weight of Neomycin A (CAS NO.3947-65-7): 322.36
The Molecular Structure of Neomycin A (CAS NO.3947-65-7):
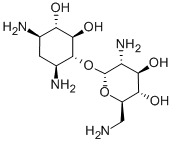
Density: 1.51 g/cm3
Flash Point: 303.3 °C
Boiling Point: 577.9 °C at 760 mmHg
Index of Refraction: 1.649
Molar Refractivity: 77.41 cm3
Molar Volume: 212.4 cm3
Polarizability: 30.69×10-24cm3
Surface Tension: 92.6 dyne/cm
Enthalpy of Vaporization: 99.33 kJ/mol
Vapour Pressure: 9.19E-16 mmHg at 25°C
Water Solubility: 1e+006(mg/L)at 25°C
Neomycin A History
Neomycin was discovered in 1949 by the microbiologist Selman Waksman and his student Hubert Lechevalier at Rutgers University. It is produced naturally by the bacterium Streptomyces fradiae
Neomycin A Toxicity Data With Reference
| 1. | scu-mus LD50:1250 mg/kg | 85ERAY Antibiotics: Origin, Nature, and Properties. 1 (1978),664. | ||
| 2. | ivn-mus LD50:320 mg/kg | 85ERAY Antibiotics: Origin, Nature, and Properties. 1 (1978),664. | ||
| 3. | ivn-mus LD50:125 mg/kg | JANTAJ Journal of Antibiotics. 27 (1974),677. |
Neomycin A Safety Profile
Poison by intravenous route. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx.
Neomycin A Specification
Neomycin A with CAS number of 3947-65-7 is also called for 2-Desoxy-4-O-(2,6-diamino-2,6-didesoxy-alpha-D-glucopyranosyl)-D-streptamin ; 4-18-00-07474 (Beilstein Handbook Reference) ; 4-O-(2,6-Diamino-2,6-dideoxy-alpha-D-glucopyranosyl)-2-deoxy-D-streptamine ; BRN 0026714 ; Dekamycin V ; Neamin ; Neamine ; Nebramycin X ; Negamicin ; UNII-5981U00LY0 . Neomycin is overwhelmingly used as a topical preparation, such as Neosporin. It can also be given orally, where it is usually combined with other antibiotics. Neomycin is not absorbed from the gastrointestinal tract, and has been used as a preventive measure for hepatic encephalopathy and hypercholesterolemia. By killing bacteria in the intestinal tract, it keeps ammonia levels low and prevents hepatic encephalopathy, especially prior to GI surgery. It has also been used to treat small intestinal bacterial overgrowth. It is not given intravenously, as neomycin is extremely nephrotoxic (causes kidney damage), especially compared to other aminoglycosides. The exception is when neomycin is included, in very small quantities, as a preservative in some vaccines - typically 0.025 mg per dose.

