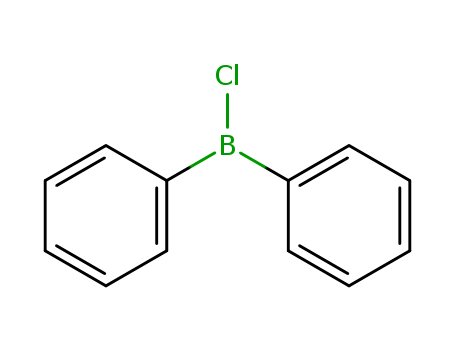
- Chemical Name:Borane, chlorodiphenyl-
- CAS No.:3677-81-4
- Molecular Formula:C12H10BCl
- Molecular Weight:200.475
- Hs Code.:
- DSSTox Substance ID:DTXSID10415984
- Nikkaji Number:J673.531B
- Wikidata:Q82225087
- Mol file:3677-81-4.mol
Synonyms:Borane, chlorodiphenyl-;diphenylchloroborane;3677-81-4;chloro(diphenyl)borane;chlorodiphenylborane;SCHEMBL2218862;DTXSID10415984;InChI=1/C12H10BCl/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10