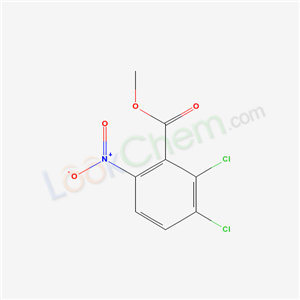
- Chemical Name:Methyl 2,3-dichloro-6-nitrobenzoate
- CAS No.:63105-60-2
- Molecular Formula:C8H5Cl2NO4
- Molecular Weight:250.0356
- Hs Code.:
- European Community (EC) Number:263-860-8
- DSSTox Substance ID:DTXSID0069672
- Nikkaji Number:J307.196K
- Wikidata:Q81996638
- Mol file:63105-60-2.mol
Synonyms:Methyl 2,3-dichloro-6-nitrobenzoate;63105-60-2;Benzoic acid, 2,3-dichloro-6-nitro-, methyl ester;EINECS 263-860-8;SCHEMBL5635837;DTXSID0069672;methyl-2,3-dichloro-6-nitrobenzoate;2,3-Dichloro-6-nitrobenzoic acid methyl ester