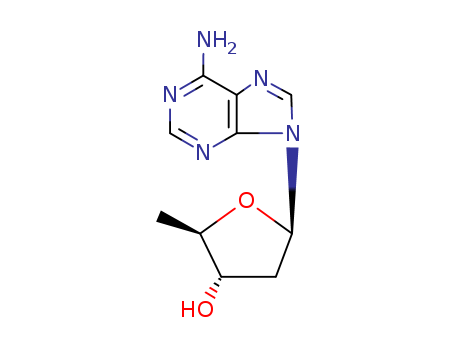
- Chemical Name:5-(6-Aminopurin-9-yl)-2-methyl-3-oxolanol
- CAS No.:6698-26-6
- Molecular Formula:C10H13N5O2
- Molecular Weight:235.246
- Hs Code.:29349990
- NSC Number:95943,95107
- DSSTox Substance ID:DTXSID90927095
- Wikidata:Q27165549
- Mol file:6698-26-6.mol
Synonyms:5-(6-aminopurin-9-yl)-2-methyl-3-oxolanol;5-(6-aminopurin-9-yl)-2-methyloxolan-3-ol;5-(6-AMINOPURIN-9-YL)-2-METHYLTETRAHYDROFURAN-3-OL;13116-42-2;NSC-95943;9-(2,5-dideoxypentofuranosyl)-9h-purin-6-amine;NSC95107;CHEMBL282503;SCHEMBL23396436;CHEBI:93820;DTXSID90927095;NSC95943;BDBM50140057;NSC-95107;AKOS032947862;PD069187;FT-0636240;BRD-A20131130-001-01-7;Q27165549;5-(6-Amino-purin-9-yl)-2-methyl-tetrahydro-furan-3-ol;2-Hydroxymethyl-5-(6-methylamino-purin-9-yl)-tetrahydro-furan-3,4-diol5-(6-Amino-purin-9-yl)-2-methyl-tetrahydro-furan-3-ol