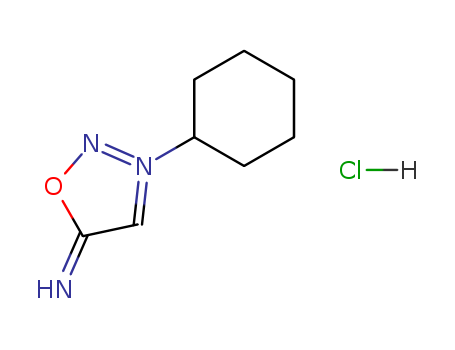
- Chemical Name:5-amino-3-cyclohexyl-1,2,3-oxadiazol-3-ium chloride
- CAS No.:29396-39-2
- Molecular Formula:C8H14 N3 O . Cl
- Molecular Weight:203.70
- Hs Code.:
- Mol file:29396-39-2.mol
Synonyms:1,2,3-Oxadiazolium,5-amino-3-cyclohexyl-, chloride (9CI); Sydnone imine, 3-cyclohexyl-, hydrochloride(7CI); Sydnone imine, 3-cyclohexyl-, monohydrochloride (8CI);3-Cyclohexylsydnonimine hydrochloride